US Pharm. 2012;37(1):HS-15-HS-18.
Movement disorders (MD) secondary to central nervous system (CNS) disease and skeletal-muscle overactivity can be extremely debilitating. In the past, many of these disorders had no effective therapy. Some may have been treated pharmacologically, with little or no response. Some were treated surgically, which was invasive and not always successful. In 1989, the first commercially available botulinum toxin (BnT) preparation was approved for the treatment of blepharospasm, an MD involving the eyelids.1,2 Since then, BnT has had clinical applications for the treatment of many other MDs, including cerebral palsy (CP), multiple sclerosis (MS), Parkinson’s disease (PD), and conditions secondary to stroke or traumatic brain injury.1 In fact, BnT has become first-line therapy for most focal dystonias (abnormal muscle tonicity affecting a single region of the body).3
Commonly Treated Movement Disorders
Some commonly treated MDs are CP, cervical dystonia (CD), blepharospasm, hemifacial spasm, laryngeal dystonia (LD), tics, and PD.
CP: CP, the most disabling childhood disorder, has an incidence of 2 to 2.5 per 1,000 live births.4 Resulting from a CNS abnormality, CP manifests as impaired movement and posture.5 Gross motor function and muscle tone are impaired.4 Spasticity is the most common manifestation of CP (70%-80%); however, dystonia may coexist, making diagnosis difficult.4,6
Dystonia occurs when agonist and antagonist muscles at a joint cocontract involuntarily, causing abnormal posture and twisting movements. In young children, toe walking and equinus gait are common manifestations; older children and adolescents more often experience weakness of the antigravity muscles, which results in different types of flexed knee gaits, including crouched gait.4
For more than 15 years, BnT has been used to treat lower-limb spasticity.4 BnT also has been shown to be safe for long-term treatment of dystonia; however, children with CP tend to experience more adverse effects from BnT than adults.3 Other treatment options include physical and occupational therapy, splinting and/or casting, surgery, and intrathecal baclofen.5,6
CD: Also known as spasmodic torticollis, CD is a neurologic disorder in which the head and neck are abnormally postured because of involuntary muscle contractions.7,8 These contractions cause pain in 70% to 90% of patients.7 The presentation of CD can be complex: There may be varying combinations of sustained and intermittent movements, and the movements may be precipitated by activities such as touching the chin or leaning back. Sitting, walking, and mental activity can precipitate abnormal movement.8 Onset typically occurs at age 40 years or older.9 CD is a lifelong condition, but spontaneous remission occurs in approximately 10% of patients. These patients end up developing CD later in life, when it ultimately becomes permanent.8
In CD, the goals of treatment with BnT are greater range of movement, reduced pain, and increased functionality.8 BnT type A (BnT A), which decreases the pain associated with CD, is an effective treatment and is considered first-line therapy.2 The mechanism by which BnT A lessens pain probably involves reducing muscle contractions and inhibiting certain peptide neurotransmitters in the area around the injection site.7 All patients should be counseled about and offered BnT. Contraindications to BnT therapy include pre-existing difficulty swallowing, underlying neuromuscular disease, and the presence of immunogenicity resulting in no response to BnT.8 Alternative treatments include physical therapy, muscle relaxants, anticholinergic drugs, and surgical denervation.8,10 Deep brain stimulation has had some promising results.2
Adverse events (AEs) occur seldom and are usually mild and self-limiting.8 They may include neck and musculoskeletal pain, muscle weakness, and injection-site pain. More serious AEs include dysphagia and respiratory, speech, and swallowing difficulties.11
Blepharospasm: This form of focal dystonia results in involuntary muscle contractions and spasms of the periocular and facial muscles, causing excessive eye blinking and sometimes sustained eye closure leading to functional blindness.2,10 Prior to BnT, there was no effective medical or surgical treatment for blepharospasm.2
BnT is considered first-line treatment for blepharospasm.10 AEs include eyelid ptosis, dry eye and mouth, diarrhea, headache, visual impairment, dyspnea, nasopharyngitis, and respiratory tract infection. BnT also may cause corneal exposure, ulceration, and ectropion.11,12
Hemifacial Spasm: Hemifacial spasm is caused by innervation of the facial nerve, which results in unilateral clonic and tonic spasms.2 Stress may exacerbate the spasms, which may persist during sleep.13 Pharmacologic therapies include carbamazepine, baclofen, and benzodiazepines, but these treatments have limited efficacy.2 BnT is the best available treatment for facial spasms.13
LD: LD, also known as spasmodic dysphonia, is a focal dystonia of the larynx. LD can present independently (e.g., Meige syndrome) or as a characteristic of other disorders (e.g., tardive dyskinesia).14 LD is uncommon, but in some cases it can contribute to mortality.15 There are two forms of LD: an adductor type (ADSD; muscles constricting inward), resulting in a strained-strangled voice, and an abductor type (ABSD; muscles constricting outward), which occurs less frequently and is characterized by a breathy hypophonic voice.2 LD may also present as a combination of ADSD and ABSD. Onset is gradual, over months or years, and is more common in women.14 Stress can exacerbate symptoms, which often subside during laughing, throat clearing, coughing, whispering, humming, and falsetto speech production.14
There are no effective therapies for LD. The American Academy of Neurology (AAN) suggests that BnT probably is effective for ADSD, but because of insufficient evidence BnT cannot be recommended for ABSD.2 Despite evidence suggesting a benefit in ADSD, many physicians are reluctant to use BnT to treat LD for fear of resulting dysphagia and/or tongue paresis.15 Further study of LD is needed, as the current literature consists of case reports and small studies.14,15
Tics: Often associated with Tourette’s syndrome, tics are brief, intermittent movements (motor tics) or sounds (phonic tics).2 Tics are preceded by a premonitory urge or sensation in the affected muscles.16 They are classified as clonic (involuntary muscle contraction and relaxation in rapid succession), tonic (continuous tension), or dystonic (involuntary tic or spasm due to disordered tonicity). Serious dystonic tics of the neck may lead to spinal disease.16
Neuroleptic drugs are often efficacious for tics, but side effects limit their use. There is anecdotal evidence that BnT has been used successfully to treat motor tics.16 The AAN has determined that, based on the literature, BnT may be effective for the treatment of motor tics, but data are insufficient to determine its efficacy for phonic tics.2
PD: Jaw tremor is common in PD and is considered an axial symptom, along with postural and gait difficulty and changes in writing. These symptoms are often refractory to levodopa and can cause the patient to experience social embarrassment. There is anecdotal evidence supporting the use of BnT for the treatment of jaw tremor secondary to PD.17
BnT
BnT is derived from Clostridium botulinum. There are seven serotypes (A-G), but only types A and B are available for clinical applications.1 BnT works by preventing the release of acetylcholine at the neuromuscular junction, resulting in a weakened or paralyzed muscle.10 BnT binds to surface receptors of the presynaptic axon and undergoes endocytosis. Once BnT is inside the cell, release of acetylcholine is inhibited and neuromuscular blockade is achieved.1,18 Collateral sprouts develop off the presynaptic terminal axon in response to the neuromuscular blockade. The collateral sprouts can establish functioning synapses within approximately 4 weeks.1,6 BnT A and BnT B exert their effects on different proteins in the axon.10 The action of BnT is not permanent, and BnT A generally has a longer duration of action than BnT B.5
Four BnT products—Botox, Dysport, Xeomin, and Myobloc—are available commercially (TABLE 1). With the exception of Xeomin and Botox, these products are not interchangeable because their potencies are different.7 To avoid confusion, the FDA has given each product a generic name. The type A formulations are onabotulinumtoxinA (Botox, Allergan), abobotulinumtoxinA (Dysport, Ipsen Biopharm), and incobotulinumtoxinA (Xeomin, Merz Pharmaceuticals). RimabotulinumtoxinB (Myobloc, Solstice Neurosciences) is the only type B formulation available in the United States.1,10
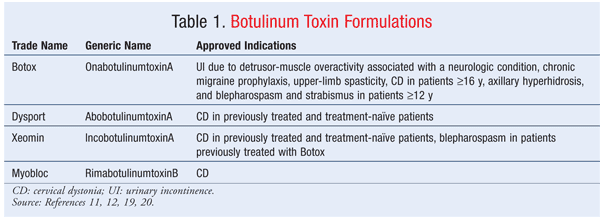
The formulations have many characteristics in common. Perhaps the most important feature of BnT prompted the FDA to mandate a black box warning about a phenomenon termed “distant spread of toxin.” Toxin can spread distally from the injection site and cause symptoms consistent with BnT effects in areas not intended. Depending upon the condition being treated and the points of injection, the following serious and sometimes life-threatening symptoms may occur: asthenia, generalized muscle weakness, diplopia, blurred vision, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence, and breathing difficulties. Symptoms may occur within hours to weeks after injection. The risk is probably greatest in children being treated for spasticity, but spasticity also can occur in adults. Elderly people and those with underlying conditions such as respiratory disorders, neuromuscular disorders, and swallowing dysfunction may also be at greater risk. These patients need to be monitored closely for AEs.11,12,19,20
AEs are dose dependent; higher doses result in a greater risk of AEs, but also in improved efficacy.8 In the event of an overdose, an antitoxin is available from the CDC; however, the antitoxin will not reverse any effects that are already apparent. The CDC may be contacted at 770-488-7100, and more information may be found at
www.cdc.gov/ncidod/srp/drugs/
Each BnT preparation contains a small amount of human albumin (e.g., 0.05% in Myobloc). The manufacturers advise that there is an extremely remote risk of viral transmission from the product to the patient.11,12,19,20 Any patient receiving a BnT product should be informed of the presence of albumin. In addition, some religions, such as the Jehovah’s Witnesses, prohibit the administration of blood products. The practitioner should respect the patient’s religious beliefs and give him or her the option of refusing BnT treatment.21
Aminoglycosides (curare-like compounds) and neuromuscular blockers or muscle relaxants can potentiate the effects of BnT, so coadministration should be avoided.11,12,19,20 Coadministration of anticholinergic drugs can potentiate the anticholinergic effects of BnT.11
With the exception of Botox, BnT is not indicated for use in children younger than 18 years.11,12,19,20 It is often used off-label for children under 18 years to treat cerebral palsy and other conditions. BnT is not recommended for pregnant or nursing women. There have been no clinical trials evaluating effects on the human fetus; however, animal studies have demonstrated that BnT is embryotoxic.11,12,19,20
Immunogenicity
Some patients develop a tolerance to BnT. The development of antibodies, which bind to the BnT molecule and prevent it from docking with the receptor, results in immunogenicity and a reduced or absent response to BnT with continuing injections.5,18,22 Immunogenicity tends to be lower with BnT A than with BnT B.3 Since BnT is highly immunogenic, its long-term efficacy is compromised.18
The original formulation of Botox (1989-1998) contained 25 ng of complex protein. The antibodies target the protein portion of the neurotoxin.23 The proteins dissociate from the BnT molecule at physiologic pH, so they have no effect on the distribution or efficacy of the toxin.24 In theory, if the protein content is minimized, the risk of immunogenicity should also be decreased. The current Botox formulation has only 5 ng of complex protein. In a study comparing the two formulations, subjects receiving the original formulation had a higher incidence of antibody development than those receiving the current formulation.23 This finding supports the theory that immunogenicity is caused by the protein portion of the BnT molecule. Factors that may lead to the development of immunogenicity are frequent injections and higher doses.12
Brands of BnT
Botox (onabotulinumtoxinA): Botox, the first commercially available BnT product, has more FDA-approved indications than the other three formulations. Botox is approved for urinary incontinence (UI) due to detrusor-muscle overactivity associated with a neurologic condition (spinal cord injury or MS) and for chronic migraine prophylaxis (³15 days/mo with headache >4 h). It also is approved for treatment of upper-limb spasticity to decrease excessive muscle tone in the elbow, wrist, and finger flexors (biceps, flexor carpi radialis, flexor carpi ulnaris, flexor digitorum profundus, and flexor digitorum sublimis muscles); CD in patients aged 16 years and older; axillary hyperhidrosis; and blepharospasm and strabismus in patients aged 12 years and older. Botox is contraindicated for UI in patients with acute urinary tract infection (UTI) or acute urinary retention.12 For UI associated with a neurologic disorder, prophylactic antibiotics (not aminoglycosides) should be given for 3 days prior to treatment, on the treatment day, and for 3 days posttreatment to guard against UTI. Dosing can be complicated and differs depending upon the indication; the package insert should be consulted.12
AEs occurring in 5% or more of patients include (depending upon the injection site) UTI, urinary retention, neck pain, headache, pain in the extremities, dysphagia, upper respiratory infection, increased cough, flulike syndrome, back pain, rhinitis, injection-site pain, nonaxillary sweating, and pharyngitis.12
Dysport (abobotulinumtoxinA): This product is indicated for CD in both treatment-naïve and previously treated patients. The initial dose is 500 U intramuscularly as a divided dose among the affected muscles. Dysport is contraindicated in patients allergic to cow’s milk protein.19
AEs occurring in ³5% of patients include muscle weakness, dysphagia, dry mouth, injection-site discomfort, fatigue, headache, neck pain, musculoskeletal pain, dysphonia, and eye disorders.19
Xeomin (incobotulinumtoxinA): Approved by the FDA in 2010, Xeomin does not contain complexing proteins, which are thought to facilitate immunoresistance to BnT.10,11,24 There have been no human studies comparing antibody development in Xeomin versus other BnT products, but animal studies have demonstrated a lack of antibody development for Xeomin.24 Xeomin is not effective in patients who are nonresponders to BnT treatment because of antibody formation.9
Xeomin is comparable to Botox in terms of efficacy and AEs.25 Xeomin and Botox have comparable potency; 1 unit of Xeomin is comparable to 1 unit of Botox.9,25 Approved indications include CD in both treatment-naïve and previously treated patients and blepharospasm in patients previously treated with Botox.11
Myobloc (rimabotulinumtoxinB): Myobloc is the only BnT B product available in the U.S., and its only approved indication is CD.20 Since Myobloc tends to be more immunogenic than BnT A formulations, chronic use of this product is limited, but it may be an option for patients who have developed immunoresistance to BnT A.8 The initial dose for previously treated patients is 2,500 to 5,000 units divided among the affected muscles; for treatment-naïve patients, the initial dose should be lower.20
Factors to Consider
When deciding whether to use BnT, the practitioner and the patient must consider certain factors. There is a possibility that BnT treatment will result in a reduction of the patient’s activity level. This must be weighed against any benefits for the caregiver. Both the patient and the caregiver must realize that BnT does not necessarily result in complete recovery of muscle function; instead, it may improve functional outcomes by reducing pain, improving range of motion, and restoring more balanced muscle function, resulting in improved muscle control.1
BnT treatment should be a multidisciplinary undertaking whenever possible. Some factors that affect the success of BnT treatment include patient goals, the severity and length of time the patient has had the disorder, symptoms, location of the movement, availability of adjunctive treatment options, and method of BnT delivery.
Conclusion
This article discussed merely a partial list of the many conditions being treated with BnT. BnT has shown some promise in the treatment of tardive dyskinesia secondary to chronic neuroleptic use.26,27 Tremor and spasticity as a result of stroke, brain trauma, MS, and CNS neoplasms are other possible indications for BnT therapy.2,6,5 The isolation and purification of BnT has led to the hope for relief from many debilitating MDs.
REFERENCES
1. Esquenazi A, Novak I, Sheean G, et al. International consensus statement for the use of botulinum toxin treatment in adults and children with neurological impairments—introduction. Eur J Neurol. 2010;17(suppl 2):1-8.
2. Simpson DM, Blitzer A, Brashear A, et al. Assessment: botulinum neurotoxin for the treatment of movement disorders (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008;70:1699-1706.
3. Albanese A, Asmus F, Bhatia KP, et al. EFNS guidelines on diagnosis and treatment of primary dystonias. Eur J Neurol. 2011;18:5-18.
4. Love SC, Novak I, Kentish M, et al. Botulinum toxin assessment, intervention and after-care for lower limb spasticity in children with cerebral palsy: international consensus statement. Eur J Neurol. 2010;17(suppl 2):9-37.
5. Simpson DM, Gracies JM, Graham HK, et al. Assessment: botulinum neurotoxin for the treatment of spasticity (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology, 2008;70:1691-1698.
6. Lundy C, Lumsden D, Fairhurst C. Treating complex movement disorders in children with cerebral palsy. Ulster Med J. 2009;78:157-163.
7. Chapman MA, Barron R, Tanis DC, et al. Comparison of botulinum neurotoxin preparations for the treatment of cervical dystonia. Clin Ther. 2007;29:1325-1337.
8. Novak I, Campbell L, Boyce M, Fung VS. Botulinum toxin assessment, intervention and after-care for cervical dystonia and other causes of hypertonia of the neck: international consensus statement. Eur J Neurol. 2010;17(suppl 2):94-108.
9. Benecke R. Xeomin in the treatment of cervical dystonia. Eur J Neurol. 2009;16(suppl 2):6-10.
10. A new botulinum toxin (Xeomin) for cervical dystonia and blepharospasm. Med Lett Drugs Ther. 2010;52:90-91.
11. Xeomin (incobotulinumtoxinA) package insert. Greensboro, NC: Merz Pharmaceuticals, LLC; July 2011.
12. Botox (onabotulinumtoxinA) package insert. Irvine, CA: Allergan, Inc; August 2011.
13. Shoaib KK, Inam-ul-Haq, Khan MD. Use of botulinum A toxin (BOTOX) in different types of facial dystonia. J Coll Physicians Surg Pak. 2009;19:742-743.
14. Truong DD, Bhidayasiri R. Botulinum toxin therapy of laryngeal muscle hyperactivity syndromes: comparing different botulinum toxin preparations. Eur J Neurol. 2006;13(suppl 1):36-41.
15. Kasravi N, Jog MS. Botulinum toxin in the treatment of lingual movement disorders. Mov Disord. 2009;24:2199-2202.
16. Agguirregomozcorta M, Pagonabarraga J, Diaz-Manera J, et al. Efficacy of botulinum toxin in severe Tourette syndrome with dystonic tics involving the neck. Parkinsonism Relat Disord. 2008;14:443-445.
17. Schneider SA, Edwards MJ, Cordivari C, et al. Botulinum toxin A may be efficacious as treatment for jaw tremor in Parkinson’s disease. Mov Disord. 2006;21:1722-1724.
18. Atassi MZ, Dolimbek BZ, Jankovic J, et al. Molecular recognition of botulinum neurotoxin B heavy chain by human antibodies from cervical dystonia patients that develop immunoresistance to toxin treatment. Mol Immunol. 2008;45:3878-3888.
19. Dysport (abobotulinumtoxinA) package insert. Wrexham, Wales: Ipsen Biopharm Ltd; April 2010.
20. Myobloc (rimabotulinumtoxinB) package insert. South San Francisco, CA: Solstice Neurosciences, Inc; May 2010.
21. Malhotra R, Huilgol SC, Selva D. Botulinum toxin and human serum albumin. Arch Ophthalmol. 2003;121:1661.
22. Atassi MZ. Immune recognition of BoNTs A and B: how anti-toxin antibodies that bind to the heavy chain obstruct toxin action. Toxicon. 2009;54:600-613.
23. Jankovic J, Vuong KD, Ahsan J. Comparison of efficacy and immunogenicity of original versus current botulinum toxin in cervical dystonia. Neurology. 2003;60:1186-1188.
24. Frevert J. Xeomin: an innovative new botulinum toxin type A. Eur J Neurol. 2009;16(suppl 2):11-13.
25. Dressler D. Routine use of Xeomin in patients previously treated with Botox: long term results. Eur J Neurol. 2009;16(suppl 2):2-5.
26. Tschopp L, Salazar Z, Micheli F. Botulinum toxin in painful tardive dyskinesia. Clin Neuropharmacol. 2009;32:165-166.
27. van Harten PN, Hovestadt A. Botulinum toxin as a treatment for tardive dyskinesia. Mov Disord. 2006;21:1276-1277.
To comment on this article, contact rdavidson@uspharmacist.com.





