US Pharm. 2016;41(2)(Specialty&Oncology suppl):13-18.
ABSTRACT: A significant amount of research has been focused on developing effective therapies in the form of cancer vaccines and treatment against cancer immune evasion. The two major categories of cancer vaccines are preventive and therapeutic. There are currently two types of FDA-approved preventive cancer vaccines (hepatitis B virus and human papillomavirus) and one therapeutic cancer vaccine (prostate cancer) available. There are also several types of cancer treatment vaccines in development that utilize immunotherapy. Immunotherapy agents constitute an additional class of drugs that use the host’s immune system to actively target tumor cells. These agents work differently from cancer vaccines by reliving the blockage of immune checkpoints utilized in certain malignancies to avoid immune recognition and destruction, targeting specific cellular pathways (CTLA-4, PD-1, and IDO receptors), and broadening the armamentarium of targeted therapies in the ongoing fight against cancer.
Understanding immune evasion as one of the new hallmark features of cancer cells has led to the successful development of immunotherapies that activate the host immune system in order to detect the tumor cells and destroy them.1 Recently, several strategies for immunotherapy, such as vaccinations, adoptive autologous transfusion of engineered immune cells, and immune reaction checkpoint activation, have made revolutionary progress to significantly improve the clinical outcome of cancer treatments.2 Cancer vaccines include two classes: preventive (prophylactic) and treatment (therapeutic).3 Traditional vaccines actively or passively bolster the immune system to fight against microbes. Both prophylactic and therapeutic cancer vaccines utilize similar mechanisms of introducing virus-like particles (VLPs) into the host so as to induce an immune response and produce a durable prevention of or effectively cure a specific malignancy.
Vaccines for Cancer Prevention
Between 15% and 25% of cancers worldwide are believed to be contributed by microbes including bacteria, viruses, and parasites.4-6 Bacterial and viral infections, such as Helicobacter pylori (H pylori ), human papillomavirus (HPV), hepatitis C virus, hepatitis B virus, human herpes virus, and HIV, have been established as major risk factors for many human cancers.4-6
Preventive vaccines are used to block development or recurrence of cancer. There are currently two types of FDA-approved cancer preventive vaccines—hepatitis B virus vaccines for liver cancer prevention and HPV vaccines for cervical and other cancer prevention (TABLE 1).7-14
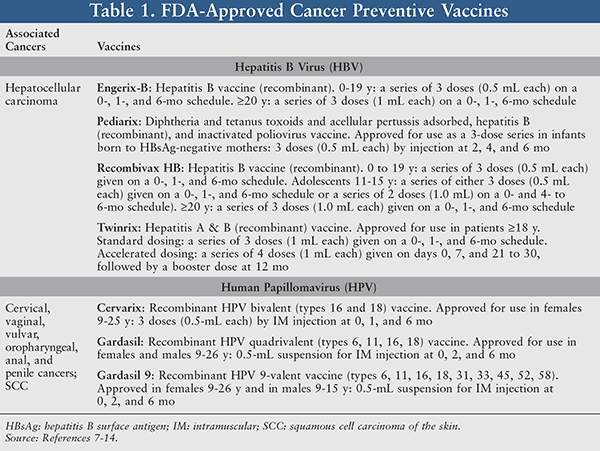
The first FDA-approved cancer vaccine was created over 30 years ago to protect against hepatitis B virus infection.15 Chronic hepatitis B infection has been identified as a major risk factor in the progression of hepatocellular carcinoma, the most common type of liver cancer. There are four hepatitis B vaccines currently available in the United States (Engerix-B, Pediarix, Recombivax HB, Twinrix; see TABLE 1).8-11 Childhood vaccination has led to a significant reduction in the incidence of hepatocellular carcinoma.15 Generally, the hepatitis B vaccines contain noninfectious hepatitis B virus surface antigen (HBsAg) produced and purified by gene engineering for intramuscular (IM) administration. Unlike the live attenuated vaccines (such as smallpox or yellow fever vaccines), which can have decades of memory, hepatitis B vaccines are subunit vaccines that only induce shorter duration memory, and booster doses are required to maintain effective immunity. Thus, most hepatitis B vaccines have a series of three doses or four doses administered within 6 months. In the clinical setting, an antibody concentration against HBsAg must be ³10 mIU/mL in order to maintain protection against the infection of hepatitis B virus.8-11,15
Gardasil and Cervarix are cancer preventive vaccines FDA-approved to protect against various types of HPV infection, which is linked to the development of many genitourinary malignancies.16 HPV can cause cervical, vaginal, vulvar, oropharyngeal, anal, and penile cancer, as well as squamous cell carcinoma of the skin. HPV causes about 70% of cervical and anal cancers worldwide, thus HPV vaccination has the potential to reduce almost two-thirds of cervical cancer deaths worldwide.5 HPVs are small DNA viruses with over 120 types being identified based on the sequence of outer capsid protein L1. Just like hepatitis B vaccines, HPV vaccines are subunit vaccines that contain noncontagious, recombinant technology-produced L1 capsid proteins of major HPV types, such as 6, 11, 16, and 18. The L1 proteins can self-assemble into noncontagious and nononcogenic VLPs to trigger innate immune reactions. Types 6 and 11 are low risk, nononcogenic HPV types involved with low-grade cervical cell abnormalities, genital warts, and laryngeal papillomas. HPV types 16 and 18 are high-risk, oncogenic HPVs responsible for about 70% of cervical cancers worldwide.16
Currently, three HPV vaccines have been approved by the FDA (TABLE 1).12-14 Cervarix is a bivalent HPV vaccine that contains L1 proteins of two high-risk HPV types (16 and 18).12 Gardasil is a quadrivalent HPV vaccine that contains L1 proteins of two low-risk HPV types (6 and 11) and two high-risk HPV types (16 and 18).13 Gardasil 9, a new 9-valent HPV vaccine that was approved by the FDA in December 2014, contains L1 proteins of HPV 6, 11, 16, 18, 31, 33, 45, 52, and 58.14 Since HPV vaccines are subunit vaccines, a series of three doses within 6 months is needed to maintain immunity.17
A vaccine for HPV types 6, 11, 16, and 18 is currently in phase II clinical trials for prevention of anal cancer and penile cancer in young HIV-positive male patients who have sex with men.18 There are also other preventive cancer vaccines currently in clinical trials. One example is a phase IIa trial for an HIV vaccine. The approach to this vaccine incorporates both T-cell and antibody responses. Some of the first HIV vaccines targeted only the antibody response approach.19
Cancer Vaccination for Treatment
In addition to the FDA-approved prophylactic vaccines, there are several therapeutic cancer vaccines currently being studied. Immunotherapy incorporates essential steps of immune response, such as the development of an antigen, activation of cancer-specific effector and cytotoxic T cells that are trafficked to the tumor site, recognition of cancer cells, and eradication of tumor cells. The cancer-immunity cycle commences with an innate “invader” response responsible for causing tumor cell apoptosis and the creation of essential antigens that are phagocytosed by the antigen-presenting cells (APCs) and presented to T cells via the major histocompatibility complex class I and II (MHC I and II) molecules. Subsequently, this activates cytotoxic T lymphocytes (CTLs), which migrate to the site of the malignant T cells. The CTLs are primed to the original antigen presented during their activation and subsequently target and eradicate the cancer cells. The methods used to develop therapeutic cancer vaccines attempt to mimic the organic immune response in one of four ways: implementing tumor-specific or nonspecific antigens; using weakened or killed cancer cells; using autologous antigen-presenting cells, such as dendritic cells (DCs); and using DNA that codes for antigens of cancer cells.20
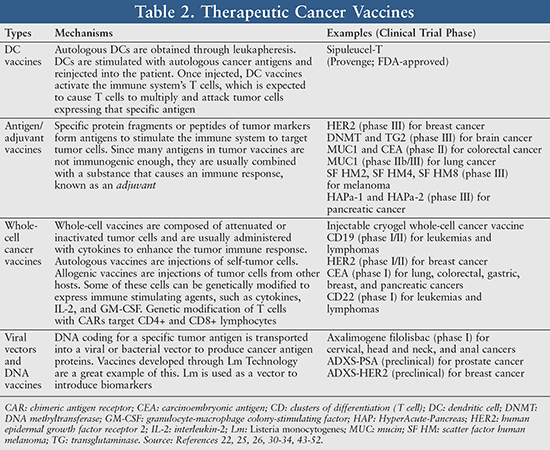
Recently, new evidence has emerged on synergistically inducing an antitumor immune response by combining cancer treatment vaccines with tumor immune checkpoint inhibitors.21 TABLE 2 summarizes types of therapeutic cancer vaccines and their respective mechanisms.
Dendritic Cell Vaccines
Sipuleucel-T (Provenge): This is the first and only cancer treatment vaccine approved by the FDA.22 Sipuleucel-T is indicated for the treatment of asymptomatic or minimally symptomatic metastatic castrate-resistant (i.e., hormone-refractory) prostate cancer.22,23
Sipuleucel-T is an example of personalized medicine, as it is manufactured using each patient’s own APCs that are activated via exposure to an antigen specific to prostate cancer. It contains autologous activated APCs that stimulate a response against PAP, an antigen expressed on most prostate cancer tissues. Once leukapheresis is completed, peripheral blood mononuclear cells are isolated, from which APC precursors, including DCs, are activated in vitro with a recombinant human fusion protein, PAP-GM-CSF (i.e., PAP linked to granulocyte-macrophage colony-stimulating factor). Once reinfused into the patient, PAP-GM-CSF targets APCs and directs the T cells to PAP, eventually destroying PAP-expressing prostate cancer cells.22
There were three recent phase III clinical trials with a similar setup that assessed the efficacy of sipuleucel-T.22-24 One showed clinical significance in overall survival, whereas the others did not indicate statistical significance. The IMPACT trial was a randomized, double-blind, placebo-controlled, multicenter trial conducted in patients with asymptomatic or minimally symptomatic metastatic castrate-resistant prostate cancer. Two randomized arms included a total of 512 patients, sipuleucel-T (n = 341) or control (n = 171). The overall survival median for sipuleucel-T was 25.8 months (95% CI, 22.8-27.7) and the control was 21.7 months (95% CI, 17.7-23.8). The hazard ratio was 0.775 (95% CI, 0.614-0.979, P = .032). The recommended course of therapy was three complete doses (IV infusions) given at 2-week intervals. The IMPACT trial is the most recent of the three phase III trials, and it supported the conclusion that sipuleucel-T prolonged survival of the participants.22-24
Antigen/Adjuvant Vaccines
NeuVax HER2 Vaccine: There is currently an ongoing multicenter, global, prospective, randomized, double-blind, controlled phase III trial (PRESENT) studying the efficacy of the nelipepimut-S (NeuVax) vaccine for the prevention of breast cancer recurrence in early-stage, node-positive breast cancer patients who have the low-to-intermediate HER2 (human epidermal growth factor receptor 2) expression gene.25 Although this vaccine prevents recurrence, it is still considered treatment because the participants have tumors with HER2 present. Enrolled patients will have tumors expressing low or intermediate levels of the HER2 protein, and the NeuVax vaccine will be given as adjuvant therapy. The study’s primary endpoint is 3-year disease-free survival (DFS).25
NeuVax is an immunodominant nonapeptide derived from the extracellular domain of the HER2 protein. The fragmented antigens from the vaccine activate the adaptive immunity, which causes CTLs to migrate to the target HER2 protein on malignant T cells and, subsequently, eradicate the tumor cells. Due to the success of the phase II trial, the FDA granted NeuVax a Special Protocol Assessment (SPA) for the PRESENT phase III trial.25,26
Whole-Cell Cancer Vaccines
Chimeric Antigen Receptors (CARs): A novel and promising approach to immunotherapy is the genetic modification of T cells with CARs. The discovery of CARs arises from the use of adoptive cellular therapy. CD4+ and CD8+ T lymphocytes are powerful components of adaptive immunity, and are essential in tumor elimination. Due to their cytotoxic capacity, T cells have emerged as attractive candidates for cancer-specific immunotherapy. First-generation CARs consist of a binding moiety that specifically recognizes a tumor cell surface antigen and a lymphocyte-activating signaling chain. The CAR-mediated recognition induces cytokine production and tumor-directed cytotoxicity of T cells. Second- and third-generation CARs include signal sequences from various costimulatory molecules resulting in enhanced T-cell persistence and sustained antitumor reaction. Clinical trials have revealed that the adoptive transfer of T cells engineered with first-generation CARs represents a feasible concept for the induction of clinical responses in some tumor patients. Further modifications, however, are required, which may be achieved by second- or third-generation CAR-engrafted T cells.27
Although the use of CARs appears promising, there are still obstacles to overcome before they can be used for a wider range of cancer types, especially because of differences in tumor microenvironments that may impact the therapy’s efficacy.28 Research and clinical trials are currently exploring the uses and benefits of modifications of T cells with CARs, including phase I and II studies in the treatment of refractory or relapsed leukemia or lymphoma.29
Viral Vectors and DNA Vaccines
Listeria monocytogenes Technology: Another example of a therapeutic cancer vaccine incorporates the use of Listeria monocytogenes (Lm) to generate an immune response to T cells directed at tumor cells. Lm Technology utilizes live, attenuated strains of Lm as a vector for the delivery of biomarkers introduced to the body. Lm is unique because it induces strong responses to MHC I and II, subsequently creating a potent CD4+ and CD8+ response. Lm’s protein, listeriolysin-O (LLO), is a major virulence factor that may exhibit a pathogen-associated molecular pattern (PAMP) and stimulate production of proinflammatory cytokines. Researchers are able to fuse genetic biomarkers to a nonfunctional truncated form of LLO and enhance immunogenicity to antigens.30,31
A preclinical study by Mkrtichyan et al used Lm Technology in combination with an anti-PD-1 (anti-programmed-death receptor 1) antibody, which increased therapeutic efficacy of LLO immunotherapy.30 This study showed significant reduction in regulatory T cells (Treg) and myeloid-derived suppressor cells (MDSC). The use of anti-PD-1 antibody showed increased antigen-specific immune response peripherally and in CD8 T cell infiltration into the tumor.30
Axalimogene filolisbac (formerly ADXS-HPV) is a therapy that uses Lm Technology immunotherapy. This vaccine targets HPV-associated cancers and is currently undergoing clinical trials as an FDA-designated orphan drug for invasive cervical, head and neck, and anal cancers.32 Two other immunotherapy vaccines currently under investigation include ADXS-PSA for use in prostate cancer and ADXS-HER2 in HER2+ solid tumors.33,34
Treatment of Immune Evasion
In the ongoing fight against cancer, new mechanisms are continually sought to gain traction against its highly variable characteristics. Just like pathogenic infections, tumor cells develop survival mechanisms, often in the form of genetic mutations that enable the cancer to evade the body’s natural defense strategies, become more prone to metastasizing, and become refractory to currently used treatments. Several key mechanisms that have been recently discovered involve tumor cell immune evasion through several cellular pathways. Of these, the most notable are the CTLA-4 (cytotoxic T-lymphocyte-associated antigen 4), PD-1, and IDO (indoleamine 2,3-dioxygenase) pathways.21
Ipilimumab (Yervoy) is a fully human monoclonal antibody modified from an immunoglobulin G1 (IgG1) molecule targeted for the CTLA-4 receptor.35 The CTLA-4 molecule serves as a coreceptor molecular checkpoint, facilitating negative regulation for activation of T cells. Binding of its ligand B7 stimulates downstream targets such as the serine/threonine phosphatase PP2A, which act to inhibit the serine/threonine kinase Akt involved in the immunostimulatory production of interleukin-2 (IL-2). This mechanism inhibits the proliferation and survival of T cells, contributing to immune evasion of tumor cells.36 Likewise, by blocking the CTLA-4 receptor, ipilimumab inhibits the immune inhibitory effects, thereby increasing the survival and proliferation of T lymphocytes and their differentiation to effector cells via the production of IL-2.37
Pembrolizumab (Keytruda) is a fully human monoclonal antibody that targets the PD-1 pathway. The PD-1 receptor is expressed on B cells and activated T lymphocytes, regulates self-tolerance, and prevents autoimmunity. PD-1 has been found to be upregulated in many tumor cells, providing a useful and plentiful target.38 By binding to its ligands, PD-L1 and PD-L2, the PD-1 receptor is involved in the downregulation of T-cell receptor signaling, proliferation, and release of proimmune cytokines. This results in T-cell anergy and cell death, which contributes to immune evasion by tumor cells.39,40 The binding of pembrolizumab to the PD-1 receptor, however, blocks inhibition of phosphatidylinositol 3-kinase (PI3K), resulting in increased accumulation of phosphorylated lipids and increased activation of Akt. This effect increases the production of IL-2, an important cytokine for T-lymphocyte proliferation and recruitment. Although mechanistically similar to the CTLA-4 pathway, the physiological target of pembrolizumab is found upstream from that of ipilimumab, and is more effective in blocking immunostimulatory signaling.41
The IDO pathway stimulates NF-IL-6/CEBP (nuclear factor for IL-6/CCAAT-enhancer-binding protein beta), which is involved in transcription regulation of immune modulators such as the immunosuppressive cytokines IL-6, IL-10, and transforming growth factor beta (TGF-. It is normally suppressed by bridging integrator 1 (BIN1); however, this mechanism is downregulated in cancer cells. Another proposed mechanism of immune escape in the IDO pathway involves inhibition of Treg cell activation through tryptophan exhaustion and kynurenine production with simultaneous inhibition of Th-17 conversion. Together, these mechanisms facilitate antigen-specific anergy among T cells and inhibit Treg recruitment, thereby enabling immune escape of tumor cells.42 Investigational small molecules such as 1-methyl-tyrptophan (1-MT), methyl-thiohydantoin, and the natural compound brassinin show promise in inhibiting cancer cells displaying increased expression of the IDO pathway11,12
CTLA-4, PD-1, and IDO pathways involved in tumor cell immune evasion are promising anticancer targets that each produce T-cell inhibition.21 Ipilimumab and pembrolizumab have similar but distinct mechanisms of action and have the same effect on IL-2. Pembrolizumab, however, is more effective because its target is more upstream.41 It appears that for many patients, immune checkpoint inhibitors alone are not enough to target the tumor, and a combination approach with therapeutic cancer vaccines would induce a more potent immune response because the cancer vaccines act by priming the T-cell response. Recently, new light has been shed on synergistically inducing antitumor immune response by combining a cancer vaccine with tumor immune checkpoint inhibitors.21 Thus far, preclinical models support this, which has led to further research and new clinical trials.
Conclusion
Recent advances in the treatment of various forms of cancer now center on using the body’s immune system to target the invading tumor. Cancer vaccines are integral members of the anticancer arsenal and are currently being implemented in the prevention of hepatitis B and HPV, in addition to being used as an effective treatment option in prostate cancer. There are also several types of therapeutic cancer vaccines in development that utilize immunotherapy. This promising field of research for cancer treatment uses different mechanisms from cancer vaccines, such as the CTLA-4, PD-1, and IDO pathways. Combining these diverse treatment modalities would serve to inhibit the mechanisms developed by many cancer types to escape the body’s natural defenses already primed by cancer vaccines, thus increasing their efficacy and improving survival rates. Therefore, more research is needed in the combined application of cancer vaccines and immunotherapy to elucidate their true clinical utility.
REFERENCES
1. Chen DS, Mellman I. Oncology meets immu-nology: the cancer-immunity cycle. Immunity. 2013;39:1-10.
2. Pardoll DM. The blockade of immune check-points in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-264.
3. Lollini PL, Cavallo F, Nanni P, Forni G. Vaccines for tumour prevention. Nat Rev Cancer. 2006;6(3):204-216.
4. Emens LA. Cancer vaccines: on the threshold of success. Expert Opin Emerg Drugs. 2008;13(2):295-308.
5. Frazer IH, Lowy DR, Schiller JT. Prevention of cancer through immunization: prospects and challenges for the 21st century. Eur J Immunol. 2007;37(suppl 1):S148-S155.
6. Mueller NE. Cancers caused by infections: unequal burdens. Cancer Epidemiol Biomarkers Prev. 2003;12(3):237s.
7. National Cancer Institute. Cancer vaccines fact sheet. National Institutes of Health. November 15, 2011. www.cancer.gov/about-cancer/causes-prevention/vaccines-fact-sheet. Accessed September 9, 2015.
8. Engerix-B (hepatitis B vaccine [recombinant]) package insert. Research Triangle Park, NC: GlaxoSmithKline; September 2015.
9. Pediarix (diphtheria and tetanus toxoids and acellular pertussis adsorbed, hepatitis B [recombinant] and inactivated poliovirus vaccine) package insert. Research Triangle Park, NC: GlaxoSmithKline; November 2013.
10. Recombivax HB (hepatitis B vaccine [recombinant]) package insert. Whitehouse Station, NJ: Merck & Co., Inc; November 2014.
11. Twinrix (hepatitis A & hepatitis B [recombinant] vaccine) package insert. Research Triangle Park, NC: GlaxoSmithKline; March 2015.
12. Cervarix (human papillomavirus bivalent [types 16 and 18] vaccine) package insert. Research Triangle Park, NC: GlaxoSmithKline; February 2015.
13. Gardasil (human papillomavirus quadrivalent [types 6, 11, 16, and 18] vaccine, recombinant) package insert. Whitehouse Station, NJ: Merck & Co., Inc; April 2015.
14. Gardasil 9 (human papillomavirus 9-valent vaccine, recombinant) package insert. Whitehouse Station, NJ: Merck & Co., Inc; December 2015.
15. Chang MH. Hepatitis B virus and cancer prevention. Recent Results Cancer Res. 2011;188:75-84.
16. National Cancer Institute. HPV and cancer. www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-fact-sheet. Accessed September 9, 2015.
17. Lowy DR, Schiller JT. Prophylactic human papillomavirus vaccines. J Clin Invest. 2006;116(5):1167-1173.
18. AIDS Malignancy Consortium. AMC-072: protective effect of quadrivalent vaccine in young HIV-positive males who have sex with males. NCT01209325. https://clinicaltrials.gov/ct2/show/NCT01209325. Accessed January 16, 2016.
19. National Institute of Allergy and Infectious Diseases. Safety of and immune response to a prime-boost vaccine regimen in HIV-uninfected vaccine-naïve adults. NCT00820846. https://clinicaltrials.gov/ct2/show/NCT00820846. Accessed January 16, 2016.
20. Anagnostou VK, Brahmer JR. Cancer immunotherapy: a future paradigm shift in the treatment of non-small cell lung cancer. Clin Cancer Res. 2015;21(5):976-984.
21. Kleponis J, Skelton R, Zheng L. Fueling the engine and releasing the break: combinational therapy of cancer vaccines and immune checkpoint inhibitors. Cancer Biol Med. 2015;12(3):201-208.
22. Provenge (sipuleucel-T) package insert. Seattle, WA: Dendreon Corporation; October 2014.
23. Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411-422.
24. Dendreon. Provenge (sipuleucel-T) active cellular immunotherapy treatment of metastatic prostate cancer after failing hormone therapy. NCT00065442. https://clinicaltrials.gov/ct2/show/NCT00065442. Accessed January 16, 2016.
25. Galena Biopharma, Inc. Efficacy and safety study of NeuVax (nelipepimut-S or E75) vaccine to prevent breast cancer recurrence (PRESENT). NCT01479244. https://clinicaltrials.gov/ct2/show/NCT01479244. Accessed January 16, 2016.
26. Mittendorf EA, Clifton GT, Holmes JP, et al. Clinical trial results of the HER-2/neu (E75) vaccine to prevent breast cancer recurrence in high-risk patients: from US Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Cancer. 2012;118(10):2594-2602.
27. Cartellieri M, Bachmann M, Feldmann A, et al. Chimeric antigen receptor-engineered T cells for immunotherapy of cancer. J Biomed Biotechnol. 2010;2010:956304.
28. Beavis PA, Slaney CY, Kershaw MH, et al. Reprogramming the tumor microenvironment to enhance adoptive cellular therapy. Semin Immunol. 2015 Nov 20 [Epub ahead of print].
29. Southwest Hospital, China. A clinical research of CAR T cells targeting cd19 positive malignant B-cell derived leukemia and lymphoma. NCT02349698. https://clinicaltrials.gov/ct2/show/NCT02349698. Accessed January 12, 2016.
30. Mkrtichyan M, Chong N, Abu Eid R, et al. Anti-PD-1 antibody significantly increases therapeutic efficacy of Listeria monocytogenes (Lm)-LLO immunotherapy. J Immunother Cancer. 2013;1:15.
31. Wallecha A, Wood L, Pan ZK, et al. Listeria monocytogenes-derived listeriolysin O has pathogen-associated molecular pattern-like properties independent of its hemolytic ability. Clin Vaccine Immunol. 2013;20(1):77-84.
32. Maciag PC, Radulovic S, Rothman J. The first clinical use of a live-attenuated Listeria monocytogenes vaccine: a phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine. 2009;27:3975-3983.
33. Hannan R, Zhang H, Wallecha A, et al. Combined immunotherapy with Listeria monocytogenes-based PSA vaccine and radiation therapy leads to a therapeutic response in a murine model of prostate cancer. Cancer Immunol Immunother. 2012;61(12):2227-2238.
34. Shahabi V, Seavey MM, Maciag PC, et al. Development of a live and highly attenuated Listeria monocytogenes-based vaccine for the treatment of Her2/neu-overexpressing cancers in human. Cancer Gene Ther. 2011;18(1):53-62.
35. Hodi FS, O’Day SJ, Mcdermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723.
36. Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25(21):9543-9553.
37. McCoy KD, Le Gros G. The role of CTLA-4 in the regulation of T cell immune responses. Immunol Cell Biol. 1999;77:1-10.
38. Poole RM. Pembrolizumab: first global approval. Drugs. 2014;74(16):1973-1981.
39. Najjar YG, Kirkwood JM. Pembrolizumab: pharmacology and therapeutics review. Am J Hematol/Oncol. 2014;10(5):17-19.
40. Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomized dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109-1117.
41. Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25(21):9543-9553.
42. Prendergast GC, Metz R, Muller AJ. Towards a genetic definition of cancer-associated inflammation: role of the IDO pathway. Am J Pathol. 2010;176(5):2082-2087.
43. Sin-chan P, Huang A. DNMTs as potential therapeutic targets in high-risk pediatric embryonal brain tumors. Expert Opin Ther Targets. 2014;18(10):1103-1107.
44. Dyer LM, Schooler KP, Ai L, et al. The trans-glutaminase 2 gene is aberrantly hypermethylated in glioma. J Neurooncol. 2011;101(3):429-440.
45. National Cancer Institute (NCI). Vaccine therapy in treatment patients with newly diagnosed advanced colon polyps. NCT02134925. https://clinicaltrials.gov/ct2/show/NCT02134925. Accessed January 12, 2016.
46. Transgene. Phase IIB/III of TG4010 immuno-therapy in patients with stage IV non-small cell lung cancer (TIME). NCT01383148. https://clinicaltrials.gov/ct2/show/NCT01383148. Accessed January 12, 2016.
47. Polynoma LLC. Study of a melanoma vaccine in stage IIb, IIc, and III melanoma patients (MAVIS). NCT01546571. https://clinicaltrials.gov/ct2/show/NCT01546571. Accessed January 12, 2016.
48. NewLink Genetics Corporation. Immuno-therapy study in borderline resectable or locally advanced unresectable pancreatic cancer (PILLAR). NCT01836432. https://clinicaltrials.gov/ct2/show/NCT01836432. Accessed January 12, 2016.
49. Bencherif SA, Warren Sands R, Ali OA, et al. Injectable cryogel-based whole-cell cancer vaccines. Nat Commun. 2015;6:7556.
50. Fuda Cancer Hospital, Guangzhou. Chimeric antigen receptor-modified T cells for breast cancer. NCT02547961. https://clinicaltrials.gov/ct2/show/NCT02547961. Accessed January 12, 2016.
51. Southwest Hospital, China. A clinical research of CAR T cells targeting CEA positive cancer. NCT02349724. https://clinicaltrials.gov/ct2/show/NCT02349724. Accessed January 12, 2016.
52. University College, London. Evaluation of CAR19 T-cells as an Optimal Bridge to Allogeneic Transplantation (COBALT). NCT02431988. https://clinicaltrials.gov/ct2/show/NCT02431988. Accessed January 12, 2016.
To comment on this article, contact rdavidson@uspharmacist.com.





