ABSTRACT: Cardiac asthma is a condition secondary to heart failure that is marked by dyspnea, wheezing, cough, frothy or bloody sputum, and rales.1 These symptoms usually occur at night and are more prevalent in the elderly population. Because its symptoms are similar to those of bronchial asthma, cardiac asthma is often misdiagnosed. However, an accurate diagnosis is imperative because treatments for the two conditions differ, and incorrect treatment can exacerbate cardiac asthma.2 IV furosemide, nitroglycerin, and morphine are used for the acute treatment of cardiac asthma.3 If the patient is hypoxic, supplemental oxygen also may be used.3 Outpatient medication regimens focus on treating heart failure.
In 1833, the physician James Hope coined the term cardiac asthma to describe the inadequate oxygenation of blood and the sensation of wanting breath resulting from it.1,4 Since then, cardiac asthma has been better defined, but there is still much confusion surrounding its diagnosis. In fact, cardiac asthma is commonly mistaken for bronchial asthma since both conditions have similar symptoms and timing.5 However, the primary mechanisms that cause these symptoms are dissimilar.5 By focusing on the subtle differences in symptoms and test results, providers can more easily differentiate the disorders.
Cardiac asthma involves paroxysms of dyspnea (breathlessness) and wheezing that often occur during the night as a result of congestion in the lungs secondary to heart failure (HF).4-6 In bronchial asthma, symptoms are due to an inflammatory process, rather than a cardiac process. It is important to correctly identify the etiology of the patient’s symptoms because treatment differs for these disorders, and incorrect treatment of cardiac asthma may exacerbate the condition.
Epidemiology
Since cardiac asthma and bronchial asthma have differing etiologies, it is pertinent to discuss epidemiological differences because the disease processes dictate the populations most affected. It makes sense that HF would be more prevalent in the elderly, given the disease progression. The American Heart Association’s 2011 update reported that HF has an incidence of 10 per 1,000 population in people older than 65 years, and the incidence of new HF events is at least 15 per 1,000.7 The incidence of new HF events can exceed 65 per 1,000 population in people older than 85 years, supporting a direct relationship between HF and age.7 Prevalence rates of HF in patients aged 20 to 30 years and those aged 80 years and older (0.3% and 11.5%, respectively) also support this relationship.7
The incidence and prevalence of cardiac asthma are unclear, owing to insufficient data from published studies. In a 2007 study, the incidence of cardiac asthma in elderly patients was 35%, versus the 10% to 15% incidence seen in younger patients in earlier studies.8 Trends noted in the cardiac asthma group (in the 2007 study) were increased use of tobacco products and diagnosis of chronic obstructive pulmonary disease.8 One factor not considered in this study was whether the wheezing was cardiac or pulmonary in origin, a distinction that could affect incidence results.8
Asthma is more prevalent in children than in adults. According to the 2009 CDC National Health Statistics report, 9.6% of children and 7.7% of adults had asthma.9 Boys were found to have a higher prevalence than girls, whereas in adulthood the opposite was true.9 In patients aged 85 years, asthma prevalence was approximately 5% in both men and women.9
The epidemiological differences between cardiac asthma and bronchial asthma reveal that cardiac asthma is more likely to occur in an older adult population, whereas bronchial asthma is more likely in children. Nevertheless, more research is necessary to clarify the incidence and prevalence of cardiac asthma.
Pathophysiology
In congestive heart failure (CHF), the heart’s inability to pump blood out of the left ventricle results in excess fluid in the pulmonary circulation.10 Pulmonary congestion is the consequence of fluid being pushed into the alveolar lumen. Cardiac asthma may be associated with bronchiolar pathology, rather than simply the accumulation of alveolar fluid.
Not only does the presence of fluid in the lungs make breathing difficult, it can cause asthmalike symptoms as well. The wheezing experienced by CHF patients may be due to a narrowing or obstruction of the bronchioles rather than simply to pulmonary congestion. Various studies have identified bronchial hyperreactivity as the mechanism behind cardiac asthma.11-13 Patients in these studies inhaled either acetylcholine or methacholine (cholinergic agonists). Compared with patients without cardiac asthma symptoms, those with symptoms demonstrated greater airway narrowing in response to the inhaled drug. The mechanism by which this narrowing occurs is not fully understood. Another study suggested that downregulation of beta2 receptors resulting from excessive adrenergic stimulation might cause bronchoconstriction in HF patients.14 Either mechanism is detrimental and undesirable in patients already experiencing pulmonary congestion.
Cardiac asthma may be better attributed to bronchial anatomy. The bronchial blood supply involves a separate vascular system from that which supplies the alveoli. Similar to the mechanism of fluid buildup in the alveoli, the blood supply to the bronchioles may exhibit fluid buildup. Two hypotheses exist regarding the pathophysiology behind this: theory A and theory B (FIGURE 1).10 Theory A hypothesizes that an increase in blood flow to the bronchial circulation may produce edema in the interstitial space, thereby squeezing the bronchiolar lumen.10 Conversely, theory B posits that certain pathological changes in HF, including pulmonary edema (PE), may compete for interthoracic space, compressing the entire airway structure and the bronchiole wall.10
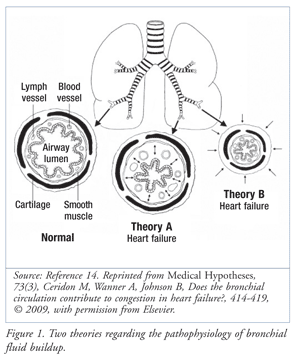
The pathologic cause of cardiac asthma is not definitively explained. Reflex bronchoconstriction involving the vagus nerve has been suggested. Increased pulmonary vascular pressure may stimulate afferent nerve endings, causing reflex narrowing of large and small airways.15 Reflex bronchoconstriction, in addition to edema and hyperreactivity, can exponentially reduce breathing capability in CHF patients. The pathophysiology of cardiac asthma may incorporate a combination of mechanisms, creating a number of treatment challenges. The absence of inflammation is the major difference between cardiac asthma and bronchial asthma, even though the symptoms are similar.
Clinical Presentation and Differential Diagnosis
As noted earlier, cardiac asthma involves symptoms of airflow obstruction that are due to HF.16 In most cases, the clinical presentation includes severe dyspnea, cough, frothy or watery sputum, and rales, but the most significant symptom indicative of cardiac asthma is wheezing.4,6 These symptoms are more prevalent in elderly patients.4
Cardiac asthma often is characterized by abrupt waking from sleep caused by sudden, severe episodes of dyspnea.4 In a typical attack, the symptoms generally subside after the patient sits upright for 20 to 30 minutes, after which he or she may be able to return to bed without needing medication.6 In severe cases, the patient may experience recurrent episodes in a single night and have cyanosis (blue or purple discoloration of the skin), cold sweats, blood-tinged sputum, and fluid buildup in the lungs.4,6 Cardiac asthma is difficult to diagnose because its symptoms are similar to those of bronchial asthma. TABLE 1 compares symptoms and diagnosing strategies for asthma, HF, and cardiac asthma.6,16-22
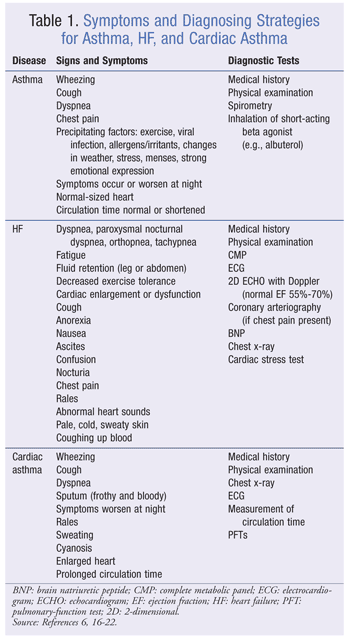
There are a few subtle differences between cardiac asthma and true asthma. Asthma often is triggered by allergens or some other precipitating factor (TABLE 1), and symptoms tend to worsen at night; cardiac asthma attacks occur almost exclusively at night when the patient is lying in bed.4 Because of preexisting heart disease, cardiac asthma patients usually have an enlarged heart (cardiomegaly) that can be seen upon chest x-ray, whereas asthma patients have a normal-sized heart.4 Severe cardiac asthma typically involves PE, which causes dyspnea, coughing, and wheezing.4 Unlike asthma, however, cardiac asthma responds poorly to bronchodilators.16
Another distinction between the two disorders is circulation time (how long it takes for blood to flow from one part of the body to another).16 In the past, circulation time typically was measured by injecting sodium dehydrocholate into an arm vein and measuring how long it took to produce a bitter taste on the tongue (arm-to-tongue circulation).23 However, this test’s unreliability caused it to be replaced by other techniques, including radionuclide angiocardiography, CT, and MRI.23 Circulation time is believed to be prolonged in HF patients; therefore, measurement may provide crucial information for differentiating between pulmonary and cardiac disorders.16,23
Since cardiac asthma stems from a heart problem, it can be difficult to distinguish its symptoms from those of HF. The three cardinal symptoms of HF are dyspnea, fatigue, and fluid retention; the presence of wheezing is diagnostic of cardiac asthma.18 Chronic HF often is associated with decreased forced expiratory volume in 1 second (FEV1); however, in one study, cardiac asthma patients (defined as having CHF and wheezing) exhibited lower FEV1 values than patients with CHF alone.8,16 Chest x-ray is useful for confirming the presence of pulmonary congestion as well as for identifying cardiomegaly, which also indicates a heart disorder.4,15 Cardiomegaly often occurs in cardiac asthma patients, but not to the same degree as in strictly HF patients.8 Physical examination and lung auscultation may reveal rales, which are attributable to PE, but the best way to diagnose HF is to obtain a two-dimensional echocardiogram with Doppler, which can identify structural abnormalities and reduced ejection fraction (EF).4,8,19
Treatment
Currently, no well-defined treatment plans exist for cardiac asthma in the acute or chronic setting. Discussions of cardiac asthma management target the pathophysiology of the underlying condition (i.e., PE and HF). The use and efficacy of bronchodilators such as albuterol and ipratropium, which are used to relieve symptoms of bronchial asthma, have not been established in cardiac asthma.8
Traditional medications used in the acute treatment of cardiac asthma include furosemide, morphine, and nitrates (TABLE 2).3,4,17 Supplemental oxygen, noninvasive ventilation (NIV), and proper positioning of the patient also are important.3 Each treatment has a unique benefit and may work synergistically with other treatments. Because patients presenting with an acute episode of cardiac asthma usually are hypoxic, the initial use of oxygen and/or NIV is recommended and has been shown to decrease mortality.24,25 If the patient shows no signs of hypoxia, oxygen and NIV are not recommended. As mentioned previously, a patient who awakens at night with dyspnea may alleviate symptoms by remaining awake for 20 to 30 minutes. Proper positioning, in which the patient stands erect or sits upright with feet hanging off the side of the bed, will result in decreased venous return.26,27 The amount of blood to the bronchioles is thereby lessened, interstitial-space edema is reduced, and the squeezing effect of the bronchiolar lumen described in theory A is diminished.3,4,26,27
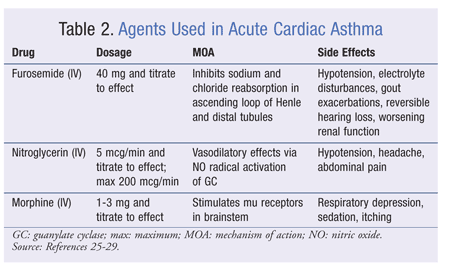
Patients experiencing an acute cardiac asthma episode likely will require fluid removal. In such cases, gradual diuresis using a loop diuretic is warranted. Furosemide, which acts at the ascending portion of the loop of Henle and at the distal tubule, inhibits the reabsorption of sodium and chloride and causes diuresis.25-27 This improves symptoms by reducing preload in the heart. Symptomatic improvement in patients with pulmonary congestion is seen with an initial recommended furosemide dosage of 40 mg IV.25-27 The dose may be increased based on clinical response but must remain gradual to avoid hypotension, electrolyte disturbances, gout exacerbations, hearing loss (reversible), and worsening renal function.24-27
Some patients may exhibit persistent pulmonary congestion despite aggressive diuresis. In these instances, an IV nitrate (e.g., nitroglycerin) may be used adjunctively in both hypertensive and normotensive patients. Acting as a venodilator, nitroglycerin will lessen the pressure in the left ventricle, thereby reducing pulmonary congestion.25,26,28 Nitroglycerin should be started at a dosage of 5 mcg/min; it may be titrated to 200 mcg/min. Nitroglycerin dosing beyond 200 mcg/min is not recommended, and the patient requiring it should be considered a nonresponder.25,26,28 Some side effects associated with nitroglycerin use are hypotension, headache, and abdominal pain.
IV morphine ameliorates symptoms in patients with pulmonary congestion.4,24 Morphine used in the setting of cardiac asthma enables easier breathing and reduces a patient’s anxiety level during the episode.4,25,26,29 The mechanism by which easier breathing occurs is believed to involve venodilation and reduction in preload.4,25,26,29 The morphine dosage commonly used is 1 to 3 mg IV every 5 minutes until relief occurs without inducing respiratory depression.24-26,29
Once the acute cardiac asthma episode is resolved, HF therapy should be initiated or optimized to prevent future occurrences. The use of ACE inhibitors and beta-blockers in HF patients is recommended to decrease morbidity and mortality rates.24,25 The continuance of diuretics postepisode will help maintain a normal volume status in this patient population and prevent future cardiac asthma occurrences. Digoxin, although not shown to reduce mortality, may be used to improve congestive symptoms in patients with HF. Additional information about chronic HF management, including anticoagulation, amiodarone use, alternative treatments, dosing, and so on, may be found in current HF practice guidelines.24,25
Conclusion
The incidence of cardiac asthma will likely increase as knowledge about diagnosis and treatment improves. Current management of cardiac asthma focuses on controlling the underlying HF and PE.5 Recognition of cardiac asthma and recommendations concerning appropriate treatment can greatly reduce disease occurrence and significantly improve the patient’s quality of life.4,7 Pharmacists can make a significant impact on the diagnosis and progression of this little-known disease. Counseling patients who are at risk for cardiac asthma and who present with related issues will enhance proper diagnosis. Pharmacists familiar with treatment options and optimization of therapy for concomitant diseases can better manage their patients’ symptoms, improve quality of life, and help slow the progression of this disorder.
REFERENCES
1. Hope J. A Treatise on the Diseases of the Heart and Great Vessels. Philadelphia, PA: Haswell and Johnson; 1842:346-365.
2. Ray P, Birolleau S, Lefort Y, et al. Acute respiratory failure in the elderly: etiology, emergency diagnosis and prognosis. Crit Care. 2006;10:R82.
3. Sabatine M. Pocket Medicine: The Massachusetts General Hospital Handbook of Internal Medicine. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2011:15.
4. Lombardo TA, Harrison TR. Cardiac asthma. Circulation. 1951;4:920-929.
5. Perlman F. Asthma and cardiac dyspnea; a differential diagnosis. Calif Med. 1951;75:199-201.
6. Hamilton JG. Cardiac asthma. Br Med J. 1955;1:39-41.
7. Roger VL, Go AS, Lloyd-Jones DL, et al. Heart disease and stroke
statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123:e18-e209.
8. Jorge S, Becquemin MH, Delerme S, et al. Cardiac asthma in elderly patients: incidence, clinical presentation and outcome. BMC Cardiovasc Disord. 2007;7:16.
9. Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005-2009. Natl Health Stat Report. 2011;Jan 12:1-14.
10. Ceridon M, Wanner A, Johnson BD. Does the bronchial circulation contribute to congestion in heart failure? Med Hypotheses. 2009;73:414-419.
11. Brunnée T, Graf K, Kastens B, et al. Bronchial hyperreactivity in patients with moderate pulmonary circulation overload. Chest. 1993;103:1477-1481.
12. Nishimura Y, Maeda H, Hashimoto A, et al. Relationship between
bronchial hyperreactivity and symptoms of cardiac asthma in patients
with non-valvular left ventricular failure. Jpn Circ J. 1996;60:933-939.
13. Nishimura Y, Yu Y, Kotani Y, et al. Bronchial hyperresponsiveness
and exhaled nitric oxide in patients with cardiac disease. Respiration. 2001;68:41-45.
14. Borst M, Beuthien W, Schwencke C, et al. Desensitization of the
pulmonary adenylyl cyclase system: a cause of airway hyperresponsiveness
in congestive heart failure? J Am Coll Cardiol. 1999;34:848-856.
15. Snashall PD, Chung KF. Airway obstruction and bronchial hyperresponsiveness in left ventricular failure and mitral stenosis. Am Rev Respir Dis. 1991;144:945-956.
16. Tanabe T, Kanoh S, Moskowitz WB, Rubin BK. Cardiac asthma:
transforming growth factor-ß from the failing heart leads to squamous
metaplasia in human airway cells and in the murine lung. Chest. 2012;142:1274-1283.
17. National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007. NIH Publication No. 08-5846.
18. Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update
incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and
Management of Heart Failure in Adults: a report of the American College
of Cardiology Foundation/American Heart Association Task Force on
Practice Guidelines: developed in collaboration with the International
Society for Heart and Lung Transplantation. Circulation. 2009;119:e391-e479.
19. Jessup M, Abraham WT, Casey DE, et al. 2009 focused update:
ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in
Adults: a report of the American College of Cardiology
Foundation/American Heart Association Task Force on Practice Guidelines:
developed in collaboration with the International Society for Heart and
Lung Transplantation. Circulation. 2009;119:1977-2016.
20. Moore TD, Anderson JR. Heart failure. In: Linn WD, Wofford MR, O’Keeffe ME, Posey LM, eds. Pharmacotherapy in Primary Care. New York, NY: McGraw-Hill Medical; 2009.
21. Kusumoto FM.Cardiovascular disorders: heart disease. In: McPhee SJ, Hammer GD, eds. Pathophysiology of Disease: An Introduction to Clinical Medicine. 6th ed. New York, NY: McGraw-Hill Medical; 2010.
22. American Heart Association. Ejection fraction heart failure
measurement.
www.heart.org/HEARTORG/Conditions/HeartFailure/SymptomsDiagnosisofHeartFailure/Ejection-Fraction-Heart-Failure-Measurement_UCM_306339_Article.jsp.
Accessed January 23, 2013.
23. Shors SM, Cotts WG, Pavlovic-Surjancev B, et al. Heart failure:
evaluation of cardiopulmonary transit times with time-resolved MR
angiography. Radiology. 2003;229:743-748.
24. Nieminen MS, Böhm M, Cowie MR, et al. Executive summary of the
guidelines on the diagnosis and treatment of acute heart failure. Eur Heart J. 2005;26:384-416.
25. Lindenfeld J, Albert NM, Boehmer JP, et al. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16:e1-194.
26. Lexi-Comp Online [database]. Hudson, OH: Lexi-Comp, Inc; 2012.
27. Furosemide. Clinical Pharmacology [database]. www.clinicalpharmacology.com. Accessed February 3, 2012.
28. Nitroglycerin. Clinical Pharmacology [database]. www.clinicalpharmacology.com. Accessed February 3, 2012.
29. Morphine. Clinical Pharmacology [database]. www.clinicalpharmacology.com. Accessed February 3, 2012.
To comment on this article, contact rdavidson@uspharmacist.com.





