US
Pharm. 2006;31(1)(Oncology suppl):16-21.
Chemoprevention, the use of
agents to delay or reverse carcinogenic progression, is an innovative research
area for head and neck cancer. Although the malignancy can be eradicated if
diagnosed in early stages, the incidence of second primary tumors (SPTs) can
threaten the long-term survival of patients with head and neck cancer. High
doses of retinoids have been studied to reverse premalignant lesions and
prevent SPTs. While it is hoped that chemopreventive research will decrease
the rate of head and neck cancer, further research is still needed to identify
agents that can prevent head and neck malignancies with minimal toxicity.
Head and neck cancer affects
approximately 3% of Americans every year. An estimated 39,250 new cases of the
disease were diagnosed in 2005, resulting in approximately 11,090 deaths.1
Head and neck cancer is more common in men and in patients older than 50
years. Several of the associated risk factors linked with head and neck cancer
include alcohol and tobacco use, betel nut consumption, frequent mouthwashing,
and exposure to human papillomavirus. The most common head and neck cancers
originate in the nasopharynx, pharyngeal wall, soft palate, tonsillar,
pyriform sinus, base of tongue, and suppraglottic larynx. When head and neck
cancer is diagnosed in early stages, patients have a better chance of
eradicating the cancer. However, in patients who present with metastatic or
recurrent disease, curative treatment is not possible, and median survival for
these patients is about six to eight months.2-4
SPTs are lesions that occur in
the genetically altered areas where the first head or neck malignancy arose.
SPTs can also be genetically independent from the initial tumor. SPTs can
threaten the long-term survival of patients who initially were cured of their
head or neck malignancy. SPTs arise at an annual incidence of 3% to 10%. In an
effort to decrease the incidence of SPTs annually, many clinicians have
pursued the arena of chemoprevention.1,2
Cancer Chemoprevention
Cancer
chemoprevention is
defined as the use of pharmacologic intervention with specific chemicals or
nutrients to suppress, revert, or prevent carcinogenic progression to invasive
cancer. This cancer control strategy is supported by the concepts of field and
multistep
carcinogenesis. Carcinogenesis is a multistep progressive pathway that results
from the collection of phenotypic and genetic variations that develop over a
period of 10 to 20 years after the initial insult (i.e., the development of
SPTs). Chemoprevention is the human intervention that halts the various steps
of the carcinogenic process over several years; preventing one or several
steps involved in carcinogenesis may delay the development of cancer.
The concept of field carcinogenesis,
initially demonstrated in the 1950s in the head and neck areas as field
cancerization, has been found to correlate to numerous epithelial sites.
Field cancerization is described as disseminated epithelial damage as a result
of exposure to inhaled carcinogens, which places large anatomic areas at risk
for development of invasive cancer. Therefore, patients who are at high risk
for developing invasive carcinoma are so at various levels, such as molecular
(i.e., gene loss or amplification), microscopic (i.e., dysplasia, metaplasia),
or gross (e.g., polyps, oral premalignant lesions) levels. Recent molecular
tests, which identify significant genetic alterations in histologically normal
tissues obtained from high-risk patients, have strongly supported this concept
of field carcinogenesis.2 For example, the mutagen sensitivity
assay tests a patient's genetic sensitivity to environmental carcinogens.
2
Chemopreventive Agents
Retinoids,
retinamides, carotenoids, cyclooxygenase-2 (Cox-2) inhibitors, and vitamin E
have been studied as chemopreventive agents for head and neck cancers.
Investigation into the field of biomarkers has also identified two novel
agents--epidermal growth factor receptor (EGFR) inhibitors and
farnesyltransferase inhibitors (FTIs), which target the EGFR and
H-ras genes, respectively. Their use as chemopreventive agents warrants
further study.2,4-6
Retinoids:
Retinoids and synthetic analogs and natural derivatives of vitamin A have
been among the most widely studied agents in human chemoprevention.3
As early as 1913, the identification of vitamin A as a vital nutrient
necessary for the basic processes of cells resulted in significant research of
the vitamin in subsequent years. In 1925, Wolbach and colleagues identified
cell histopathologic changes associated with higher risk of precancerous
lesions due to vitamin A deficiency, leading to the discovery of retinols and
the naturally occurring retinoid derivatives.3 Epidemiologic
studies evaluating vitamin A ingestion and cancer risk in the 1970s revealed
computed indices of total vitamin A intake with a decreased risk of lung
cancer.3
Vitamin A is a vague term that
includes two large families of dietary factors known as the preformed vitamin
A (mainly retinyl esters and retinal/retinol) and provitamin A carotenoids
(e.g., beta-carotene, other carotenoids that are precursors of retinol).
Preformed vitamin A is found primarily in foods derived from animals, whereas
provitamin A carotenoids are commonly found in fruits and vegetables.3
Retinoids are necessary for
vital processes such as cell growth, differentiation, and death. Several
retinoids, such as vitamin A, have been shown to suppress or revert
epithelial carcinogenesis and prevent the progression of various carcinomas,
including those of the skin, lung, bladder, oral cavity, and esophagus, in
animal models. Retinoids exert activity in the promotion and progression
phases of carcinogenesis that are significant for chemoprevention in humans.
Single-chemical activity has been demonstrated with natural retinoids (e.g.,
13-cis retinoic acid [13-cRA], 9-cis retinoic acid [9-cRA], and retinyl
palmitate) and synthetic retinoids (e.g., fenretinide, N-retinamide).
In 1985, Sporn and colleagues redefined retinoids as substances that bind and
stimulate one or more receptors, which can lead to the initiation of a
biologic response.3
The molecular mechanism of
action of retinoids is very similar to that of the steroid hormones in that
the retinoid nuclear receptors are part of the steroid receptor superfamily.
Two distinct receptors, RAR and RXR, as well as their respective subtypes,
distinguish the retinoid receptors from the steroid receptors. These retinoid
receptors are DNA-binding transcription components that can stimulate or
suppress the gene expression necessary for the cell growth, differentiation,
and death. Evaluation of retinoid receptor expression patterns in cancerous,
precancerous, and normal tissues reveals the importance of retinoids in cancer
progression. However, the exact action through which vitamin A or its
derivatives exert their chemopreventive activity is still unclear.2,3,5
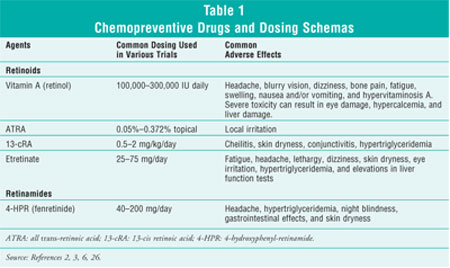
Specific agents and their respective dosing schemas in chemoprevention trials
are listed in table 1.
Carotenoids:
Dietary beta-carotene has been associated with reduced cancer rates and is one
of the most commonly studied carotenoids.2 Animal models have
demonstrated inhibition of oral malignancy with topical beta-carotene.
Epidemiologic study findings have led to further investigation of
beta-carotene in phase II and III clinical trials. Furthermore, beta-carotene
has proven to possess some activity in oral premalignancy. Early trials have
shown that oral leukoplakia responds to beta-carotene at rates as high as 44%
to 71%. Adverse effects may include diarrhea, skin discoloration, and in rare
cases, ecchymoses and arthralgias.2,5-7
Cox-2 Inhibitors: Inhibition
of cox-2 activity has been the focus of several chemoprevention methods.2
One approach to altering carcinogenesis is inhibiting the up-regulation of
prostaglandin synthesis in precancerous and cancerous tissue. The theory is
that cyclooxygenase catalyzes the production of prostaglandin and mutagenic
electrophiles. Prostaglandin levels have been identified in many epithelial
cancers, including those of the head and neck, at elevated concentrations.
Prostaglandins alter the proliferation of cells and promote angiogenesis, the
development of new blood vessels.
The two isoforms of
cyclooxygenase are known as cox-1 and cox-2. The overexpression
of cox-2 in epithelial cells prevents apoptosis (cell death), resulting in an
increase of neoplastic potential of activated cells. Cox-2 is believed to
augment the development of vascular growth factors (resulting in
neoangiogenesis, the development of new blood vessels located in the tumors)
and regulate cytokines involved in chronic inflammation (leading to epithelial
carcinogenesis). The overexpression of cox-2 is nearly 100-fold greater in
head and neck cancer tissue than in normal tissue. Cox-2 inhibitors have been
proven to prevent the development of colonic polyps in patients with familial
adenomatous polyposis. Possible prevention of other carcinomas, such as
hepatocellular and those of the breast and bladder, has also been suggested.
In addition, chronic inflammation is linked to an increased chance of
developing epithelial malignancy. Such data warrant the further investigation
of cox-2 inhibitors as an effective chemopreventive strategy. However, due to
the recent data reported in the APPROVe trial that demonstrate an association
between rofecoxib and an increased risk of cardio-vascular events, many of the
cox-2 inhibitor chemopreventive trials have been arrested and are being
reevaluated.2,5,8,9
Vitamin E:
Vitamin E has a vital role in the enzymatic initiation of hematopoiesis,
pollutant detoxification, and drug metabolism. Epidemiologic and laboratory
trials have demonstrated the anticarcinogenic activity of vitamin E. Its
mechanism of action of antioxidant activity is believed to be related to its
antioxidant properties. Topical vitamin E has effectively decreased
progression of oral cancer in animal study models.2,10
A recent meta-analysis
evaluating the use of vitamin E in various trials revealed that high doses of
the vitamin (>150 IU/day) may increase mortality from any cause and should be
avoided.11 These findings have halted numerous current vitamin E
chemoprevention trials and warrant further investigation.
EGFR Inhibitors:
The EGFR is a transmembrane protein important in the proliferation and
survival of cancer cells. Overall, the overexpression of EGFR on cancer cells
leads to the proliferation and sustained growth of these cells, resulting in
shorter disease-free intervals and shorter overall survival. Up-regulation of
the EGFR and its ligand, tumor growth factor–alpha, occurs early in the
multistep development of head and neck carcinogenesis and therefore occurs
more frequently with advanced stages of dysplasia. In addition, EGFR
overexpression in premalignant lesions tends to be a sensitive indicator of
carcinogenic potential.
Tyrosine kinase inhibitors,
small-molecule inhibitors of EGFR, are an attractive option for prevention of
head and neck cancers due to their relatively mild adverse-effect profile
(e.g., skin rash, diarrhea) and availability as an oral formulation. However,
rare cases of interstitial pneumonitis have been documented with these agents,
which is a potential concern for their use in the chemopreventive setting.
12,13 Erlotinib and gefitinib are examples of oral EGFR tyrosine kinase
inhibitors (TKIs).2,10
FTIs:
Metastasis and tumor invasion and development have been aided by the
low-molecule-weight guanine triphosphates (i.e., ras, RhoA, Rac-1, and
Cdc42). Members of the ras gene family are the most frequently altered
protooncogenes found in various tumor types. Ras mutations are believed to
occur in approximately 30% of all human cancers.2 Since ras is
believed to exert its neoplastic activity via farnesylation, FTIs have been
established to prevent this process.2,5,6
p53 Gene
Tumor-suppressor
gene p53 is located on chromosome 17. Overexpression of p53 has
been related to increased progression of mild to severe dysplasia to invasive
carcinoma. Expression of the tumor suppressor p53 gene on head and neck
cancer cells has proven to be indicative of shorter survival and may be useful
in identifying individuals who are at higher risk for developing recurrent
carcinoma or SPTs.2,5,6
Clinical Trials
Leukoplakia and
erythroplakia are oral lesions directly associated with cancer development and
thus are considered excellent research areas for development of
chemoprevention. Leukoplakia is defined as a white patch in the oral cavity
that cannot be removed by scraping and cannot be classified clinically or
histologically as any other definable lesion. Oral leukoplakia occurs in about
0.1% to 0.2% of the general population and transforms into carcinoma in about
2% to 3% of these cases.2,10 The natural course of leukoplakia is
unpredictable, with spontaneous improvement arising in several cases.
Erythroplakia is a red,
nonelevated plaque located on the oral or pharyngeal surfaces. The condition
is associated with a higher risk for subsequent malignant transformation and
is commonly associated with in situ or invasive cancer. Leukoplakia and
erythroplakia are frequently correlated with tobacco use (e.g., cigarette
smoking, tobacco chew, snuff) and betel quid chewing. Alcohol abuse is an
additional risk factor for these conditions. The progression of leukoplakia
and erythroplakia to invasive cancer depends mainly on degree of dysplasia,
clinical features of the lesions, and minimal improvement in the resolution
rate of these lesions after smoking cessation.2,3,10
Since surgical excision of
multiple extensive lesions cannot cure or prevent the development of new
lesions, other treatment interventions such as chemopreventive measures should
be sought.2 Oral leukoplakia lesions can be safely followed
histologically and clinically. Most importantly, oral premalignant lesions
serve as markers of broad areas of injury and increased risk for cancer
progression elsewhere in the aerodigestive system. Consequently, testing of
oral premalignancy has been vitally important for cancer chemoprevention.
Numerous agents, such as retinoids, have been researched over the years and
have shown promising results for the treatment of head and neck cancer.2
Trials in Oral Premalignancy
Retinoid Trials:
A placebo-controlled double-blind study examined 44 patients using high-dose
13-cis retinoic acid (13-cRA, 1 to 2 mg/kg/day) for three months.14
The clinical response rate, measured as a major reduction in the size of the
oral leukoplakia, was 67% in the retinoid group compared to 10% in the placebo
group (P=.0002). Patients were followed up to six months. The study
demonstrated two key points: the toxicities associated with high-dose 13-cRA
were frequent and severe, and remission was brief, such that within three
months of therapy cessation, more than half of the patients experienced a
relapse. Toxicities associated with high-dose 13-cRA include cheilitis, facial
erythema, conjunctivitis, skin dryness, and hypertriglyceridemia.14
A second trial was designed to
address the severe toxicities and short-lived remissions with use of high-dose
13-cRA.15 In this study, 53 patients received induction therapy
with high-dose 13-cRA (1.5 mg/kg/day) followed by randomization to maintenance
therapy for nine months. Patients whose lesions had responded or remained
stable with induction therapy were then randomized to maintenance therapy with
either low-dose 13-cRA (0.5 mg/kg/day) or low-dose beta-carotene (30 mg/day).
The beta-carotene group was utilized due to low toxicity associated with
beta-carotene and results from positive uncontrolled trials. The induction
therapy had produced a clinical response rate in 55% of patients, similar to
the results of the earlier trial. All patients were assessable for maintenance
therapy. The 13-cRA group demonstrated 33% further lesion reduction, as
compared to 10% in the beta-carotene group. The rate of disease progression
after or during administration of low-dose 13-cRA therapy was only 8% compared
to 55% with beta-carotene therapy (P<.001). Although the severity of
adverse effects was reduced significantly with low-dose 13-cRA, the reduction
was greater in the beta-carotene group, which had minimal adverse effects.
15
Additional studies were
conducted to evaluate the combination of retinoids with beta-carotene.
However, due to the negative findings of beta-carotene on lung cancer
incidence in the large lung cancer chemoprevention studies (i.e.,
Alpha-Tocopherol/Beta-Carotene Trial and the Beta-Carotene and Retinol
Efficacy Trial), beta-carotene inclusive trials in oral leukoplakia patients
who smoked were stopped early.
A study examined 65 patients
with oral leukoplakia resulting from chewing betel nut quid or tobacco.16
Patients were randomized to receive vitamin A 100,000 IU twice weekly or a
placebo for six months. Remission was achieved in 57% of the vitamin A group,
compared to 3% of the placebo group.16 There was no evidence of
disease progression in patients receiving vitamin A therapy, while progression
did occur in 21% of patients receiving placebo.2,16 Various doses
of vitamin A and its derivatives have been studied, but the optimal dosing
schema and duration with minimal adverse effects has yet to determined.
Nonretinoid Trials:
Several trials have evaluated the use of various agents as chemopreventive
measures. For example, one study evaluated the effects of beta-carotene in 130
patients with oral leukoplakia in a three-group trial (i.e., beta-carotene,
placebo, and beta-carotene plus retinol).6 The results demonstrated
that the combination of retinol and beta-carotene was more likely to induce
remission than beta-carotene alone.6
An attenuated adenovirus that
selectively targets the p53 gene was evaluated in a mouthwash
(ONYX-015) in 22 patients with oral leukoplakia.17 Patients were
scheduled into three different cohorts: 1010 plaque-forming units
(pfu) daily for five days every four weeks, 1010 pfu every week for
24 weeks, or 1011 pfu daily for five days followed by weekly
administration over the next five weeks. Patients were asked to hold the viral
mouthwash in their mouths for at least 30 seconds; many patients found this
task quite difficult. Histologic resolution of dysplasia was observed in 37%
of patients, but a majority of the responses were temporary. Adverse effects
were mild in nature. Further studies need to be conducted to elucidate
activity of the p53 gene in the treatment of oral dysplasia.17
A randomized, double-blind,
placebo-controlled study assessed the use of a ketorolac tromethamine oral
rinse in 57 patients with oropharyngeal leukoplakia to analyze the reduction
in lesion size.18 Patients were instructed to hold 10 mL of a 0.1%
ketorolac solution or 10 mL of a placebo solution in their mouths for at least
30 seconds twice daily for 90 days. Both rinses were well tolerated with
minimal adverse effects. There were no significant differences between the
rinses in regards to reduction of lesion size.18
Adjuvant Chemoprevention
Trials for Prevention of SPTs:
A randomized, placebo-controlled, phase III chemoprevention trial evaluated
103 previously treated patients with head and neck cancer, stages I to IV
(oral cavity, pharynx, larynx).19 Patients were randomized to
receive high-dose 13-cRA (50 to 100 mg/m2 daily) or placebo for one
year. There were no major differences in the number of local, regional, or
distant recurrences of the initial carcinoma between the two groups. However,
the 13-cRA group had a substantially lower number of SPTs. Due to increased
toxicity, 33% of patients did not finish the 12-month treatment plan. At
median follow-up (32 months), 4% of patients in the 13-cRA group had one or
more SPTs, compared to 24% in the placebo group (P=.005). As with previous
trials, adverse effects such as cheilitis, skin dryness, conjunctivitis, and
hypertriglyceridemia were reported with high-dose 13-cRA. At a 54.5-month
follow-up (long-term), the development rate of SPT was 14% in the 13-cRA
group, compared to 31% in the placebo group (P=.04).19
A second study was conducted
in an effort to decrease toxicity by utilizing a low dose of retinoids.20
The study, which included 1,191 patients with a history of head and neck
cancer, randomized patients to receive either placebo or 13-cRA at 30 mg/day
for three years. The long-term results demonstrated that while low-dose 13-cRA
did not alter the rate of SPT development, it may be able to delay recurrence
rates.10
The Euroscan trial assessed
2,592 patients who had been definitively treated for their primary tumors (40%
lung cancer and 60% head and neck cancer) and randomized them receive either
retinyl palmitate, N-acetylcysteine, both agents, or placebo for two
years.21 The results of this trial did not show any survival
benefit or decrease in SPTs with retinyl palmitate, N-acetylcysteine,
or a combination of the two.21
In a separate study, 316 patients
with a previous history of definitively treated head and neck cancer were
randomized to receive placebo or etretinate adjuvantly.22 Results
of the study did not demonstrate a survival benefit, change in disease-free
survival rate, or change in the development rate of SPTs in either group.
Adverse effects included cheilitis, conjunctivitis, and dermatologic changes
such as cutaneous rash, pruritis, and erythema.22
Biochemoprevention:
Biochemoprevention treatment was developed for patients whose premalignant
lesions are resistant to single-agent retinoid treatment and are at high risk
of progressing to carcinoma. Biochemoprevention uses a combination of agents,
usually retinoids, interferon, and alpha-tocopherol. A nonrandomized study
evaluated this triple-drug regimen (isotretinoin 80 mg/m2/day,
interferon-alpha 3 million units/m2 twice weekly, and
alpha-tocopherol 1,200 IU/day) for one year in 36 patients with premalignant
lesions, mostly in the laryngeal areas or oral cavity.23 The study
showed that biochemoprevention was effective for laryngeal lesions but not for
oral cavity lesions. Based on these results, another trial is currently under
way to evaluate induction therapy using biochemopreventive agents for one year
followed by maintenance therapy consisting of fenretinide, a retinamide,
versus placebo for two years.23,24
Another biochemoprevention
trial was conducted in patients with locally advanced head and neck cancer.
25 Patients received 13-cRA, interferon, and alpha-tocopherol for one
year (isotretinoin 50 mg/m2/day; interferon-alpha 3 million units/m
2 three times weekly; alpha-tocopherol 1,200 IU/day). At 24 months
(median), 14% of patients developed recurrent disease and 86% of the patients
completed the yearlong treatment. One patient developed an SPT. Overall
survival at one year was 98% and at two years was 91%. Adverse effects
included mild to moderate mucocutaneous effects, fatigue, flu-like symptoms,
anorexia, hypertriglyceridemia, and peripheral neuropathy. A randomized phase
III trial is currently under way to confirm these phase II results.25
Role of the Pharmacist
When patients inquire about the use of vitamin therapy for the prevention of
cancer in doses higher than recommended by the FDA, pharmacists should
encourage them to consult with their physician prior to initiation. Higher
doses of vitamin therapy are associated with increased side effects. If
patients are not closely monitored by a health care professional, the adverse
effects can be detrimental.
If a patient is currently
receiving a chemopreventive agent mentioned in this article, the pharmacist
should encourage patient adherence. For example, high doses of retinoid
therapy are associated with problematic adverse effects, and nonadherence to
therapy is very possible. Since this therapy is recommended for high-risk
patients for possible prevention of
invasive carcinoma, adherence is vital.
Conclusion
Chemoprevention is
an exciting and promising area for the management of head and neck cancer.
High doses of retinoids as single agents have shown activity against oral
leukoplakia. However, the adverse effects associated with high-dose retinoids
(i.e., , facial erythema, conjunctivitis, skin dryness, and
hypertriglyceridemia) may result in increased nonadherence. Concerns regarding
the ideal dosing and duration of retinoid therapy as a single agent or in
combination with other agents still remain.
Molecular targeted agents such
as FTIs, cox-2, and TKIs are an important area of research for future clinical
trials since head and cancers represent a progressive genetic disorder. The
effectiveness of biochemoprevention using triple therapy consisting of
isotretinoin, interferon, and vitamin E needs to be proven through larger
trials. In addition, acceptable toxicity with new agents and good patient
compliance need to be addressed in future chemoprevention trials. While great
strides have been made in understanding the multistep processes of head and
neck carcinogenesis, the area of chemoprevention warrants more extensive
clinical research.
REFERENCES
1. Cancer
statistics 2005. Available at: www.cancer.org. Accessed August 31, 2005.
2. Harrison LB,
Sessions RB, Hong WK. Head and Neck Cancer: A Multidisciplinary Approach
. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2004:985-1000.
3. DeVita VT, Hellman
S, Rosenberg SA. Cancer: Principles & Practice of Oncology. 6th ed.
Philadelphia: Lippincott Williams & Wilkins; 2001:575-590.
4. Kim ES, Hong WK. An
apple a day: does it really keep the doctor away? The current state of cancer
chemoprevention. J Natl Cancer Inst. 2005;97:468-470.
5. Papadimitrakopoulou
VA. Chemoprevention of head and neck cancer: an update. Curr Opin Oncol
. 2002;14:318-322.
6. Rhee JC, Khuri FR,
Shin DM. Advances in chemoprevention of head and neck cancer. Oncologist
. 2004;9:302-311.
7. Natural Standard
Database. Beta-carotene monograph. Available at: www.naturalstandards.com.
Accessed August 31, 2005.
8. FDA Public Health
Advisory: Safety of Vioxx. Available at: www.fda.gov/
cder/drug/infopage/vioxx/PHA_vioxx.htm. Accessed August 31, 2005.
9. Bresalier RS,
Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in
a colorectal adenoma chemoprevention trial. N Engl J Med.
2005;352:1092-1102.
10. Tsao AS, Kim ES,
Hong WK. Chemoprevention of cancer. CA Cancer J Clin. 2004;54:150-180.
11. Miller ER 3rd,
Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E
supplementation may increase all-cause mortality. Ann Intern Med.
2004;142:37-46.
12. Package insert.
Tarceva (erlotinib). Melville, NY: OSI Pharmaceuticals. Available at:
www.gene.com/gene/products/information/pdf/tarceva-prescribing.pdf. Accessed
December 6, 2005.
13. Package insert.
Iressa (gefitinib). Wilmington, DE: AstraZeneca Pharmaceuticals LP. Available
at: www.astrazeneca-us.com/pi/iressa.pdf. Accessed December 6, 2005.
14. Hong WK, Endicott
J, Itri LM, et al. 13-cis-retinoic acid in the treatment of oral leukoplakia.
N Engl J Med. 1986;315:1501-1505.
15. Lippman SM,
Batsakis JG, Toth BB, et al. Comparison of low-dose isotretinoin with beta
carotene to prevent oral carcinogenesis. N Engl J Med. 1993;328:15-20.
16. Stich HS, Hornby
AP, Mathew B, et al. Response of oral leukoplakias to the administration of
vitamin A. Cancer Lett. 1988;40:93-101.
17. Rudin CM, Cohen
EEW, Papadimitrakopoulou VA, et al. An attenuated adenovirus, ONYX-015, as
mouthwash therapy for premalignant oral dysplasia. J Clin Oncol.
2003;21:4546-4552.
18. Mulshine JL,
Atkinson JC, Greer RO, et al. Randomized, double-blind, placebo-controlled
phase IIB trial of the cyclooxygenase inhibitor ketorolac as an oral rinse in
oropharyngeal leukoplakia. Clin Cancer Res. 2004;10:1565-1573.
19. Hong WK, Lippman
SM, Itri LM, et al. Prevention of second primary tumors with isotretinoin in
squamous-cell carcinoma of the head and neck. N Engl J Med.
1990;23:795-801.
20. Khuri FR, Kim ES,
Lee JJ, et al. The impact of smoking status, disease stage, and index tumor
site on second primary tumor incidence and tumor recurrence in head and neck
retinoid chemoprevention trial. Cancer Epidemiol Biomark Prevent.
2001;10:823-829.
21. van Zandwijk N,
Dalesio O, Pastorino U, et al. EUROSCAN, a randomized trial of vitamin A and
N-acetylcysteine in patients with head and neck cancer or lung cancer. For the
European Organization for Research and Treatment of Cancer Head and Neck and
Lung Cancer Cooperative Groups. J Natl Cancer Inst. 2000;92:977-986.
22. Bolla M, Lefur R,
Ton Van J, et al. Prevention of secondary primary tumors with etretinate in
squamous cell carcinoma of the oral cavity and oral pharynx. Results of a
multicentric double-blinded randomized study. Eur J Cancer.
1994;30A:767-772.
23. Papadimitrakopoulou
VA, Claymin GL, Shin DM, et al. Biochemoprevention for dysplastic lesions of
the upper aerodigestive tract. Arch Otolaryngol Head Neck Surg.
1999;125:1083-1089.
24. Shin DM, Mao L,
Papadimitrakopoulou VM, et al. Biochemopreventive therapy for patients with
premalignant lesions of the head and neck and p53 gene expression. J Natl
Cancer Inst. 2000;92:69-73.
25. Shin DM, Khuri FR,
Murphy B, et al. Combined interferon-alpha, 13-cis-retinoic acid,
alpha-tocopherol in locally advanced head and neck squamous cell carcinoma;
novel bioadjuvant phase II trial. J Clin Oncol. 2001;19:3010-3017.
26. Natural Standard
Database. Vitamin A monograph. Available at: www.naturalstandards.com.
Accessed August 31, 2005.
To comment on this article,
contact
editor@uspharmacist.com.





