ABSTRACT: The management of infection caused by nontuberculous mycobacteria (NTM) can present a hardship for patients and a challenge for clinicians, due to variation in the abilities of clinical laboratories to identify causative organisms; inducible antibiotic resistance among many mycobacterial isolates; long courses of sometimes toxic antimicrobial agents; and the potential for incomplete bacterial eradication with disease relapse. The pharmacist can play a critical role in assisting with antibiotic selection and monitoring the effectiveness of drug therapy for patients with NTM infections, particularly when treatment options are limited to difficult-to-obtain agents such as clofazimine.
USPharm. 2020:45(5)(Specialty& Oncology suppl):9-12.
Infections due to nontuberculous mycobacteria (NTM) are relatively uncommon and are historically associated with patients who have an acquired, inherited, or pharmacologically induced immunocompromised state; who have hematologic cancers (such as leukemia); or who have chronic lung disease.1 Recently, the incidence of infections due to NTM has been on the rise, with an observed increase in pulmonary and skin infections in both immunocompromised and nonimmunocompromised individuals; these are now regarded as an emerging public health threat.2-6
These infections are often protracted, with long-term sequelae common among patients, particularly in those with complicated or deep-seated skin-structure infections that often require surgical intervention for resolution.2,3
Antimicrobial regimen selection can be highly specific based on the mycobacterial species and can be confounded by the limitations of clinical laboratories in identifying a recovered organism beyond the genus or group level. Furthermore, many of the most effective medications for treating serious NTM infections, such as aminoglycosides, are associated with unacceptable or intolerable adverse effects that can limit their utility in the often prolonged courses of treatment.2,3,7 A pharmacist involved in the management of the patient with serious NTM infections should be aware of potential nonstandard alternatives to primary treatment regimens.
Nontuberculous Mycobacteria
NTM are ubiquitous, acid-fast bacteria found worldwide in soil and water. There are currently more than 120 identified species of NTM, with more than half of these recognized as pathogens.3,7,8 Of these NTM, the rapidly growing mycobacteria (RGM) are some of the most commonly implicated in pulmonary and cutaneous infections.2 Mycobacterium chelonae, Mycobacterium abscessus group, and Mycobacterium fortuitum are generally considered to be the most important of these, with M abscessus being responsible for approximately 80% of pulmonary disease due to RGM and M chelonae and M fortuitum responsible for the majority of RGM-related extra-pulmonary infections.2,7,9 M chelonae has been identified as a cause of cutaneous and deep-tissue infections in immunocompetent and immunocompromised patients related to contaminated water sources, trauma, and surgery.3 A recent multistate outbreak of NTM infections was identified in which administration of improperly stored vaccines led to more than 100 injection-site infections.10
Identification of NTM to the level of speciation may present a challenge for many clinical laboratories. M chelonae and M abscessus historically have been identified as the M chelonae abscessus group without further differentiation.11 As understanding of the pathology of these organisms evolves, greater specificity in organism identification is required to provide effective treatment for patients, yet the availability of technology to provide this level of speciation remains inconsistent. The need for more detailed identification becomes apparent when considering that, unlike M chelonae, some subspecies of M abscessus are known to harbor an inducible erythromycin ribosome methylation (erm) gene that can render macrolide antibiotics, considered first-line treatment for NTM infections, ineffective.8,12
Antibiotic Regimens
Regimens for the treatment of rapidly growing NTM infections should be tailored to the mycobacterial species and adjusted for the individual isolate’s sensitivity pattern whenever possible. With mild infections, particularly those of a cutaneous nature, oral therapy may be a reasonable option. Monotherapy should generally be avoided due to the high potential for the development of resistance, especially when the individual speciation and sensitivity reports are not available.2,8 Macrolides such as clarithromycin are considered core therapeutic options, but if M abscessus subspecies abscessus or M abscessus bolletii are identified, or if M abscessus cannot be ruled out as the infecting organism, a repeat culture with sensitivity should be obtained at a minimum of 2 weeks after initiation of a macrolide to assess for inducible macrolide resistance.2,8,13 Other oral medications that may have activity against RGM include fluoroquinolones such as moxifloxacin, sulfonamides, linezolid, doxycycline, and clofazimine. At least two agents for at least 4 months should be considered for uncomplicated infections.2,8,14
With complex or severe disease or an infectious process that does not resolve with oral agents, parenteral antibiotics are recommended. Effective agents include aminoglycosides such as amikacin (or tobramycin with M chelonae infections), imipenem, cefoxitin, linezolid, and tigecycline. A multidrug parenteral regimen for a minimum of 6 months is often required for severe disease. For infections due to isolates with complex resistance patterns or for patients who cannot tolerate certain antibiotics due to adverse effects or hypersensitivities, combinations of oral and parenteral choices may be required. An infectious-disease specialist consultation should be considered for any patient with complex NMT infection whenever possible.2,15-17
Clofazimine Pharmacology
Clofazimine is described as a phenazine dye that has weak bactericidal activity against various mycobacterial species. Since 1982 it has been considered by the World Health Organization to be a first-line agent in the treatment of Hansen’s disease (leprosy), the causative agent of which is Mycobacterium leprae, and more recently, as a Group B second-line agent for the treatment of multidrug-resistant tuberculosis.4,18,19 Additionally, it exhibits good activity against various NTM such as M chelonae, M abscessus group, and M fortuitum.4,20,21
The mechanism of action of clofazimine is not well understood, but the drug’s antibacterial activity may be mediated in part by its ability to bind mycobacterial DNA.8,22 Alternatively, it has been postulated that clofazimine may interact with the bacterial cell membrane to disrupt cellular integrity and function.4,23,24
Absorption of clofazimine after oral ingestion is highly variable, with estimates ranging from 45% to nearly 70%. Taking clofazimine with food may increase absorption and improve tolerability.4,8,18,25
Clofazimine is considered to be highly lipophilic and accumulates readily in macrophages and adipose tissue, which contributes to the drug’s notably long half-life, estimated to be from 10 to 70 days. It is distributed extensively throughout the adrenals, bone marrow, heart, kidneys, liver, lungs, and pancreas, and it concentrates well in skin lesions. Time to steady state has been postulated to be up to 70 days, but this is mostly theoretical, with little scientific evidence in support.4,8,23,25
Metabolization of clofazimine is minimal and primarily occurs by glucuronidation in the liver; the major route of excretion is biliary. Additionally, a small amount of clofazimine is excreted in urine as well as sputum, sweat, and breast milk.8,25,26
There are several noteworthy adverse effects associated with clofazimine, the most prominent of which may be discoloration of skin, hair, conjunctivae, and body fluids such as tears, sputum, sweat, urine, and feces. This discoloration has been noted to occur in up to 75% to 100% of patients receiving clofazimine and is described as reddish to brownish black or orange-pink, occurring more commonly in patients with fair skin or upon exposure to sunlight. Though considered to be at least partially reversible, it may take months to years after discontinuation of therapy to resolve; there have been some reports of permanent discolorations.8,18,23,26 Disturbingly, this skin discoloration has been linked to adverse psychological effects including severe depression and suicide; the manufacturer recommends monitoring for depression or suicidal ideation while patients are receiving treatment.18,26
The package insert contains a warning regarding QT prolongation and torsades de pointes. As with other medications that carry this warning, the potential for QT prolongation increases when receiving multiple QT-prolonging drugs, and an ECG should be monitored regularly with discontinuation of therapy occurring if the QTc interval reaches or exceeds 500 ms.26
Other adverse effects include gastrointestinal (GI) discomfort—abdominal and epigastric pain, nausea, vomiting, and diarrhea. Less commonly, bowel obstruction, GI bleeding, and hepatic symptoms have occurred. Some patients have reported irritation of the eyes, phototoxicity, and, rarely, neurologic symptoms including dizziness, drowsiness, headaches, and neuralgias.26
Obtaining Clofazimine
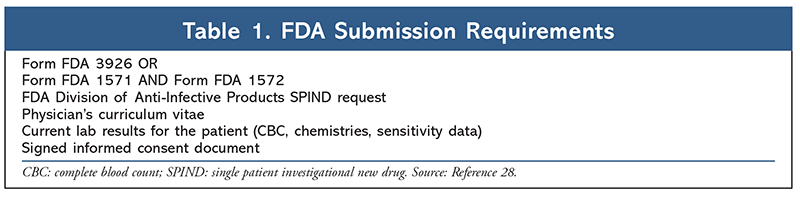
Clofazimine is FDA approved only for the treatment of leprosy and is not commercially available in the United States. The process involved with obtaining the drug can be confusing, particularly since the process has undergone recent changes. Providers requiring patient access for clofazimine must typically go through one of two channels. For patients with leprosy, the National Hansen’s Disease Program (NHDP) in Baton Rouge, Louisiana, maintains the investigational new drug application. A physician is required to register as an investigator with the NHDP, and upon approval for use, the medication is dispensed from NHDP to the prescriber.27 For all off-label uses, physicians must submit a request for a single patient investigational new drug directly to the FDA (TABLE 1).28
Once approved by the FDA, additional treatment information must be provided to the Novartis Pharmaceuticals Managed Access Program (MAP) (TABLE 2) which, upon approval, will dispense the medication directly to the prescribing physician. It is advisable to contact the FDA and Novartis Pharmaceuticals Corporation early in the process to confirm the specific requirements and forms that must be completed prior to approval of use and to inquire about any new changes in the process (TABLE 3). The Novartis MAP submission process changed to use of the Grants, External Studies, and Managed Access System portal in December 2019. The planned daily dosage, expected duration of therapy, and a plan for patient monitoring for both resolution of infection and for adverse effects should be considered prior to submission of materials.29
Upon dispensation of clofazimine, counseling emphasizing adverse effects as well as contact information for questions or concerns during treatment should be provided. During the course of treatment, the patient should be assessed regularly for physical and psychological changes.
Conclusion
Infections due to rapidly growing nontuberculous mycobacterial organisms are becoming more prevalent. The diagnosis and treatment of these infections can present a challenge due to difficulty in identifying specific pathogens and selecting effective antimicrobial therapy in the face of variable resistance patterns. Patients may require long durations of treatment and experience poor outcomes with long-term or permanent sequelae from the infectious process as well as adverse effects from antimicrobials and possibly surgical interventions. The pharmacist is uniquely suited to provide counseling for patients regarding the identification and management of medication-related adverse effects as well as monitoring for medication interactions that could potentially decrease the efficacy of antibiotic therapy or lead to medication-potentiated harm. Additionally, the pharmacist can be a resource in the selection and procurement of difficult-to-manage antimicrobials such as clofazimine.
REFERENCES
1. Adjemian J, Olivier KN, Seitz AE, et al. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med. 2012;185(8):881-886.
2. Philips RC, Hoyer PE, White SM, et al. Cutaneous nontuberculous mycobacteria infections: a retrospective case series of 78 patients from the Texas Gulf Coast region. J Am Acad Dermatol. 2019;81(3):730-739.
3. Gonzalez-Santiago TM, Drage LA. Nontuberculous mycobacteria: skin and soft tissue infections. Dermatol Clin. 2015;33(3):563-577.
4. McGuffin SA, Pottinger PS, Harnisch JP. Clofazimine in nontuberculous mycobacterial infections: a growing niche. Open Forum Infect Dis. 2017;4(3):ofx147.
5. Obregon-Henao A, Arnett KA, Henao-Tamayo M, et al. Susceptibility of Mycobacterium abscessus to antimycobacterial drugs in preclinical models. Antimicrob Agents Chemother. 2015;59(11):6904-6912.
6. Brown-Elliot BA, Nash KA, Wallace RJ Jr. Antimicrobial susceptibility testing, drug resistance mechanisms, and therapy of infections with nontuberculous mycobacteria. Clin Microbiol Rev. 2012;25(3):545-582.
7. Shen Y, Wang X, Jin J, et al. In vitro susceptibility of Mycobacterium abscessus and Mycobacterium fortuitum isolates to 30 antibiotics. Biomed Res Int. 2018;2018:4902941.
8. Mandell GL, Bennett JE, Mandell DR, Dolin R. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Churchill Livingstone/Elsevier.
9. Kasperbauer SH, De Groote MA. The treatment of rapidly growing mycobacterial infections. Clin Chest Med. 2015;36(1):67-78.
10. Blau E. A series of unfortunate events–how one company’s mishandling of vaccine led to a Mycobacterium fortuitum outbreak in three U.S. States. 2019; CDC Epidemic Intelligence Service. www.cdc.gov/eis/conference/dpk/Mishandling_of_Vaccine_Led_to_Outbreak.html. Accessed April 8, 2020.
11. Simmon KE, Brown-Elliott BA, Ridge PG, et al. Mycobacterium chelonae-abscessus complex associated with sinopulmonary disease, northeastern USA. Emerg Infect Dis. 2011;17(9):1692-1700.
12. Brown-Elliott BA, Vasireddy S, Vasireddy R, et al. Utility of sequencing the erm(41) gene in isolates of Mycobacterium abscessus subsp. abscessus with low and intermediate clarithromycin MICs. J Clin Microbiol. 2015;53(4):1211-1215.
13. Esteban J, Garcıa-Pedrazuela M, Munoz-Egea MC, Alcaide F. Current treatment of nontuberculous mycobacteriosis: an update. Expert Opin Pharmacother. 2012;13(7):967-986.
14. Halpern J, Biswas A, Cadwgan A, Tan BB. Disseminated cutaneous Mycobacterium chelonae infection in an immunocompetent host. Clin Exp Dermatol. 2010;35(3):269-271.
15. Wallace RJ Jr, Brown BA, Onyi GO. Skin, soft tissue, and bone infections due to Mycobacterium chelonae: importance of prior corticosteroid therapy, frequency of disseminated infections, and resistance to oral antimicrobials other than clarithromycin. J Infect Dis. 1992;166(2):405-412.
16. Stone MS, Wallace RJ Jr, Swenson JM, et al. Agar disk elution method for susceptibility testing of Mycobacterium marinum and Mycobacterium fortuitum complex to sulfonamides and antibiotics. Antimicrob Agents Chemother. 1983;24(4):486-493.
17. Swenson JM, Wallace RJ Jr, Silcox VA, Thornsberry C. Antimicrobial susceptibility of five subgroups of Mycobacterium fortuitum and Mycobacterium chelonae. Antimicrob Agents Chemother. 1985;28(6):807-811.
18. Legendre DP, Muzny CA, Swiatlo E. Hansen's disease (leprosy): current and future pharmacotherapy and treatment of disease-related immunologic reactions. Pharmacotherapy. 2012;32(1):27-37.
19. WHO consolidated guidelines on drug-resistant tuberculosis treatment. Geneva: Switzerland. World Health Organization; 2019. www.who.int/tb/publications/2019/ consolidated-guidelines-drug-resistant-TB-treatment/en/. Accessed April 22, 2020.
20. Shen GH, Wu BD, Hu ST, et al. High efficacy of clofazimine and its synergistic effect with amikacin against rapidly growing mycobacteria. Int J Antimicrob Agents. 2010;35(4):400-404.
21. Singh S, Bouzinbi N, Chaturvedi V, et al. In vitro evaluation of a new drug combination against clinical isolates belonging to the Mycobacterium abscessus complex. Clin Microbiol Infect. 2014;20(12):1124-1127.
22. Levy L, Shepard CC, Fasal P. Clofazimine therapy of lepromatous leprosy caused by dapsone-resistant Mycobacterium leprae. Am J Trop Med Hyg. 1972;21(3):315-321.
23. Cholo MC, Steel HC, Fourie PB, et al. Clofazimine: current status and future prospects. J Antimicrob Chemother. 2012;67(2):290-298.
24. Cholo MC, Mothiba MT, Fourie B, Anderson R. Mechanisms of action and therapeutic efficacies of the lipophilic antimycobacterial agents clofazimine and bedaquiline. J Antimicrob Chemother. 2017;72(2):338-353.
25. Holdiness MR. Clinical pharmacokinetics of clofazimine: a review. Clin Pharmacokinetics. 1989;16(2):74-85.
26. Lamprene (clofazimine) prescribing information. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016.
27. Health Resources and Services Administration. National Hansen's Disease (Leprosy) Program. www.hrsa.gov/hansens-disease/index.html. Accessed April 9, 2020.
28. FDA. For physicians: how to request single-patient expanded access (“compassionate use”). www.fda.gov/drugs/investigational-new-drug-ind-application/physicians-how-request-single-patient-expanded-access-compassionate-use. Accessed April 9, 2020.
29. Novartis. Managed access programs. www.novartis.com/our-focus/healthcare-professionals/managed-access-programs. Accessed April 9, 2020.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.