ABSTRACT: Dupuytren’s disease is a fibroproliferative disorder characterized by deformity of the hand because of fibrosis. Patients may present with difficulty extending their hand, or in advanced cases, they may be unable to place their hand flat on a tabletop. Dupuytren’s disease has no known cure; therefore, the goal of treatment is to correct the contracture through nonsurgical, minimally invasive, and surgical methods.
Dupuytren’s contracture is a flexion deformity of the hand caused by progressive fibroproliferation.1 Within weeks to months, nodules and palpable cords develop on the fascia, the fibrous layer of tissue that lies underneath the skin in the palm and fingers. These nodules and cords cause flexion contractures in which the joint of the finger cannot be straightened actively or passively.2 Although finger contractures were first observed over 400 years ago, widespread recognition of the underlying disease did not occur until the 1830s after work by French surgeon Baron Guillaume Dupuytren.3
Epidemiology
Due to a high prevalence (30%) of the disease in white Northern European and Norwegian males aged 60 years and older, Duputyren’s disease has often been referred to as “Vikings” or “Nordic” disease.4 However, there is no evidence the disease originated in the Scandinavian population. The prevalence increases with age, from 12% in adults aged 55 years and older to 29% in those aged 75 years and older.5 Initially, it was thought Dupuytren’s disease only occurred in men; however, that has been disproven with a current estimated male-to-female ratio of approximately 6:1.4
Genetics
A genetic component to Dupuytren’s disease shows that Northern European descent increases the likelihood of acquiring the disease.3 Additionally, the inheritance is thought to be autosomal dominant, with one being three times more likely to develop Dupuytren’s disease if a sibling has the condition. While there has not been a single gene identified, disfunction of numerous genes may cause disruption in the ability to regulate the formation and breakdown of collagen.3 A genome-wide association study in 960 Dutch individuals with Dupuytren’s disease identified 11 single-nucleotide abnormalities from nine different genetic loci.6 Additionally, six of the nine loci identified also contain genes for the regulation of fibroblast proliferation and differentiation. Interference with these genes could alter fibroblast function and cause the formation of nodules or cords in the fascia of the hand.6.
Pathophysiology
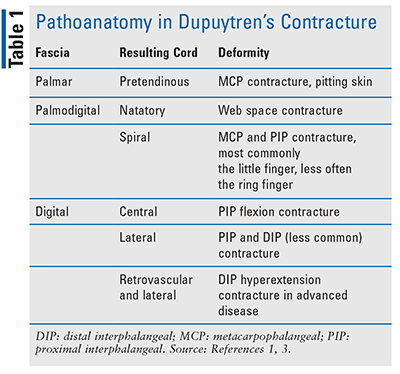
Dupuytren’s contracture progression results from diseased fascial bands (or aponeurosis), which may be damaged through repetitive trauma that is commonly associated with manual labor.3 Repeated fascial trauma, along with other risk factors described below, promotes the release of free radicals, which stimulates interleukin-1 release, an inflammatory cytokine responsible for transforming growth factor beta (TGF-beta). This promotes myofibroblast proliferation and the production of type III collagen rather than collagen type I found in healthy fascia. With continued trauma, nodules form on and around the fascia; over time, type III collagen attaches to and connects the nodules forming cords on top of and around the fascia. The nodules and cords constrict the fascia and may extend into and restrict extension of the distal digits, causing the deformation known as Dupuytren’s contracture.3 The most common joints involved are the metacarpophalangeal (MCP) joint or the knuckle, followed by the proximal interphalangeal (PIP) joint, and the distal interphalangeal (DIP) joint, which is rarely affected.1 TABLE 1 denotes the fascia and cords involved.
Risk Factors and Clinical Presentation
Risk factors for Dupuytren’s disease include repetitive trauma to the hand, such as with manual labor, excessive alcohol intake, smoking, epilepsy, diabetes, age, and genetics, including male sex.1,3 Clinical presentation may differ significantly depending upon early or advanced disease.3 The third through fifth digits (the thumb is digit 1 and the pinky, digit 5) are the most affected, and in the early-stage disease, patients may present with pitting or thickening of the skin. As firm, painless nodules develop along the internal fascia of the palm, the patient may notice progressive flexion contracture (a bent joint that cannot be straightened), which is more common in advance disease. Moreover, clinical presentation varies based on the fascia and digits involved.3
With advanced disease, collagen attaches to the nodules, forming thick cords that contract the digits and deform the hand.1,3 Cords extending up the fingers may be visible and cause extensive hand contraction. While most common in the palm of the hands, Dupuytren’s disease can affect other areas commonly exposed to trauma, such as the feet.3 In advanced disease, patients may not be able to lay their hand flat on a table. Advanced progression may also cause Garrod nodes (knuckle pads over the dorsal PIP joint). These patients may develop Ledderhose disease (plantar fibromatosis) or Peyronie’s disease (penile fibromatosis).3
Diagnostic Criteria and Methods
Dupuytren’s disease is typically diagnosed based on the patient’s presentation, patient history, and any effect on activities of daily living.1 Hueston’s tabletop test is used to evaluate disease progression and can indicate the need for corrective measures. The test is considered positive for Dupuytren’s when the patient cannot lay the hand flat on a table, indicating greater than 30° of MCP contracture.1
Staging and Classification
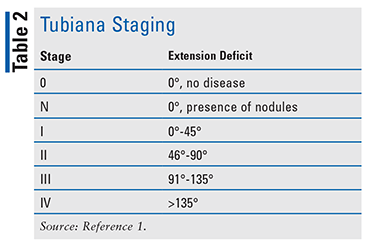
Different types of classification and staging are available for Dupuytren’s disease.1 Luck’s classification, based on the cellular process of disease progression, is broken into the proliferative, involutional, and residual stages. The proliferative stage indicates large myofibroblasts with immature fibroblasts; the involutional stage denotes a dense network formed by the myofibroblasts and a higher ratio of collagen type III to collagen type I; and in the residual stage, an extensive matrix of dense collagenous tissue has formed with fibroblasts as the predominant cell type.1 Disease severity is assessed using the Tubiana staging system (see TABLE 2).1
Treatment
Treatment options for Dupuytren’s disease are not curative but are symptomatic, and they primarily include operative and nonoperative measures to correct the contracture.7 Management is patient-specific and uses a stepwise approach based on disease severity, risk of complications, concern for recurrence, and cost. Percutaneous needle aponeurotomy or collagenase Clostridium histolyticum injections can be considered in mild-to-severe disease, with more invasive therapies such as percutaneous needle fasciotomy or a limited fasciotomy reserved for advanced disease or rapid reoccurrence after initial treatment. Collaborative treatment plans should always be the goal, with patients aware of the progressive nature of the disease.
Procedure-based modalities are used in the setting of symptomatic contractures, and surgery has been considered the standard of care in the treatment of Dupuytren’s contracture.7 With the advancement in interventions and multiple treatment modalities to choose from, disease severity should guide which procedure the patient receives. Additionally, the recurrence rate after procedures, risk of complications, and adverse events should be factored into the decision.7
Nonsurgical Options
Collagenase Clostridium histolyticum Injections: Collagenase C histolyticum injections are FDA-approved for treatment of Dupuytren’s contracture in adults with a palpable cord.8 The pharmaceutical contains two clostridial collagenases that hydrolyze collagen acting as an enzymatic fasciectomy. Treatment involves injecting directly into cords that run through the affected joint.9 The fingers are then manually manipulated to further sever the cord, releasing the contracture.8 The dose varies with the joint, with the MCP joint necessitating 0.25 mL and the PIP joint 0.20 mL.8
The JOINT I and JOINT II phase III clinical trials evaluated the safety and efficacy of collagenase C histolyticum injections in adult patients with Dupuytren’s contracture who had a fixed-flexion contracture of >20°.9 The primary endpoint reduction of contracture to within £5° of full extension was achieved in 497 of 879 (57%) joints, with 292 joints demonstrating a response within 7 days. Additionally, 369 (70%) contractures at the MCP joint and 129 (37%) at the PIP joint achieved clinical success. Of note, 625 (71%) joints did not require a second injection, and 780 (89%) did not require a third. The most common adverse effects include peripheral edema, contusion, bruising, injection-site pain, skin tenderness, lymphadenopathy, and pruritus.8 Treatment-emergent complications occurring in <1% of patients include complex regional pain syndrome, flexor tendon rupture, and anaphylaxis.3,8
Triamcinolone Acetonide Injections: Triamcinolone acetonide injections have also been studied for use in Dupuytren’s contracture. First, the area is pretreated with a local anesthetic such as lidocaine.10 Next, triamcinolone is injected directly into the nodules, inhibiting TGF-beta-1 and decreasing fibroblast activity.11 Triamcinolone acetonide injections offer the benefit of limited recovery time compared with surgical measures.11
Triamcinolone acetonide injections were studied as primary treatment in 63 patients (60% male sex; average age 55 years) with Dupuytren’s nodules.10 After an average of 3.2 injections per nodule, 97% of patients experienced a reduction in nodule size by approximately 60% to 80%. However, within 1 to 3 years, 50% of patients experienced recurrence of disease with nodule growth requiring additional triamcinolone acetonide treatments.10 Additionally, triamcinolone acetonide injections as adjuvant treatment after a needle aponeurotomy (described below) showed a lower total active extension deficit at 6 months that lasted up to 24 months compared with needle aponeurotomy without triamcinolone injections. However, after 24 months the differences between groups ceased.12
Surgical Options
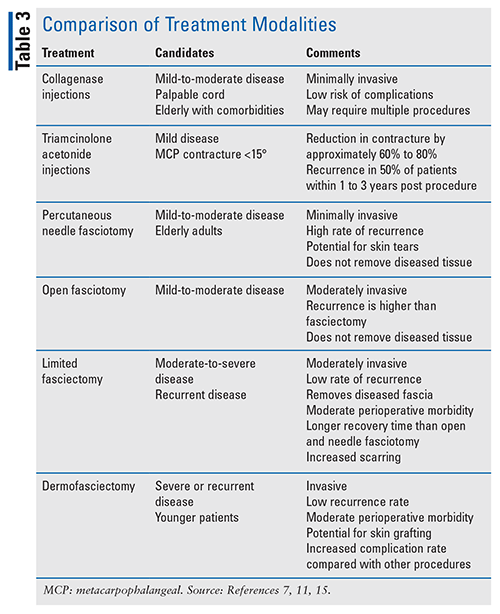
Surgical intervention, which is generally indicated in patients with ³30° of MCP contracture or any PIP contracture (usually >15°), involves fascial excision either as fasciotomy (division of cord to release contracture, without cord removal), or fasciectomy (removal of diseased fascia, including cord and nodule) with excision of the diseased fascia (limited) or a skin graft (dermofasciectomy).1,3 MCP joints are more likely than PIP joints to respond to surgery.3 Percutaneous needle aponeurotomy fasciotomy has a higher reoperation rate than limited fasciectomy or dermofasciectomy; however, there are fewer complications overall.1 Patients may undergo more than one procedure, which may or may not have been the primary procedure. See TABLE 3, which compares treatment modalities.
Fasciotomy: Percutaneous needle fasciotomy (also known as percutaneous aponeurotomy or needle fasciotomy) is a minimally invasive procedure performed on multiple contractures during the same session in which a hypodermic needle is repeatedly inserted into the cord at multiple positions.1,3 The corresponding finger is manually manipulated until maximal passive extension occurs. Since it can be performed in an office setting under local anesthesia, patients with small, palpable cords in the MCP and PIP joints are good candidates. Results from a retrospective study of 52 patients (56 joints) showed 77% improved extension in MCP and PIP joints, but most contractures (65%) reoccurred within 3 to 4 years.13 Another study comparing needle fasciotomy to limited fasciectomy (see below) reported recurrence rates much lower with the latter, 84.9% versus 20.9%.14 Complications with needle fasciotomy are minimal but may include nerve damage, skin lacerations, and flexor tendon injuries.
During an open fasciotomy, cords are incised with a scalpel using direct vision, which protects neurovascular structures because they are less likely to be damaged.15 Following the procedure, recovery typically occurs within several weeks with minimal complications. Results from a retrospective study of 1,077 patients (865 men and 212 women) receiving an open fasciotomy showed that 93% of affected joints achieved completed release; 13.5% needed a second operation at an average of 46 months following the initial procedure.15
Fasciectomy: A limited fasciectomy is the most performed operation for Dupuytren’s contracture.3,11 Due to the invasive nature of the procedure, general or regional anesthesia is often required.11 Patients are encouraged to flex and extend the fingers as soon as anesthesia wears off.13 It is effective for patients with moderate-to-severe disease and has shown a success rate of 94% with MCP joints and 46% with PIP joints.7,14 Recurrence rates of 23% to 38% have been reported.1,14 Additionally, patients who underwent limited fasciectomy reported significantly higher satisfaction scores than patients who received collagenase injection and lower recurrence rates than with needle fasciotomy.14,16
The most aggressive procedure is a dermatofasciectomy.7 Typically, dermatofascietomy is reserved for those with advanced disease or who have failed other procedures. Younger patients are also candidates.1 In a study with 143 procedures (103 patients, 83% male, average age 69.2 years), dermatofasciectomy had a 5.8-year follow-up recurrence rate of 11.6%.17 Prolonged rehabilitation and additional postoperative complications are associated with both procedures.7
Role of the Pharmacist
Although Dupuytren’s disease is typically diagnosed and treated by a patient’s primary care provider, pharmacists play a role in both pretreatment and posttreatment care. Pharmacies serve as health education centers for their communities, and pharmacists can answer any questions and refer patients when cords or nodules are present. The pharmacist may also recommend risk-mitigation techniques such as blood sugar management or smoking cessation. Finally, pharmacists can recommend posttreatment care, including wound management, scar-prevention methods, and comfort techniques, including protective gloves or OTC pain management.
Conclusion
Despite being first identified over 400 years ago and causing significant deformity in patients, Dupuytren’s disease has no known cure. Patients may present with a deformity of the hand, known as Dupuytren’s contracture. For patients experiencing a Dupuytren’s contracture, the goal of treatment is to correct the contracture utilizing nonsurgical, minimally invasive, and surgical methods. Multiple treatment methods may be required and repeated multiple times throughout a patient’s life, with reoccurrence of contracture as early as 6 months following treatment. Pharmacists are typically considered the most accessible healthcare professional, may be the first to identify contractures, and can refer patients to their primary care providers, when necessary. Additionally, pharmacists can assist patients with postoperative care and encourage risk-mitigation techniques. While the disease has no known cure, current treatment methods offer patients symptomatic relief and improved quality of life.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
1. Dutta A, Jayasinghe G, Deore S, et al. Dupuytren’s contracture—current concepts. J Clin Orthop Trauma. 2020;11(4):590-596.
2. Lanting R, Broekstra DC, Werker PMN, van den Heuvel ER. A systematic review and meta-analysis on the prevalence of Dupuytren disease in the general population of Western countries. Plast Reconstr Surg. 2014;133(3):593-603.
3. Black EM, Blazar PE. Dupuytren disease: an evolving understanding of an age-old disease. J Am Acad Orthop Surg. 2011;19(12):746-756.
4. Hindocha S, McGrouther DA, Bayat A. Epidemiological evaluation of Dupuytren’s disease incidence and prevalence rates in relation to etiology. Hand (N Y). 2009;4(3):256-269.
5. Dolmans GH, Werker PM, Hennies HC, et al. Wnt signaling and Dupuytren’s disease. N Engl J Med. 2011;365(4):307-317.
6. Li W, Ahn AC. Subcutaneous fascial bands—a qualitative and morphometric analysis. PLoS One. 2011;6(9):e23987.
7. Mella JR, Guo L, Hung V. Dupuytren’s contracture: an evidence based review. Ann Plast Surg. 2018;81:(6S suppl 1):S97-S101.
8. Xiaflex [package insert]. Dublin, Ireland: Endo Pharmaceuticals Inc; 2021.
9. Witthaut J, Jones G, Skrepnik N, et al. Efficacy and safety of collagenase Clostridium histolyticum injection for Dupuytren contracture: short-term results from 2 open-label studies. J Hand Surg Am. 2013;38(1):2-11.
10. Ketchum LD, Donahue TK. The injection of nodules of Dupuytren’s disease with triamcinolone acetonide. J Hand Surg Am. 2000;25(6):1157-1162.
11. Denkler KA, Vaughn CJ, Dolan EL, Hansen SL. Evid
ence-based medicine: options for Dupuytren’s contracture: incise, excise, and dissolve. Plast Reconstr Surg. 2017;139(1):240e-255e.
12. McMillan C, Binhammer P. Steroid injection and needle aponeurotomy for Dupuytren disease: long-term follow-up of a randomized controlled trial. J Hand Surg Am. 2014;39(10):1942-1947.
13. van Rijssen AL, Werker PM. Percutaneous needle fasciotomy in Dupuytren’s disease. J Hand Surg Br. 2006;31(5):498-501.
14. van Rijssen AL, ter Linden H, Werker PMN. Five-year results of a randomized clinical trial on treatment in Dupuytrenʼs disease: percutaneous needle fasciotomy versus limited fasciectomy. Plast Reconstr Surg. 2012;129(2):469-477.
15. Stewart CJ, Davidson DM, Hooper G. Re-operation after open fasciotomy for Dupuytren’s disease in a series of 1,077 consecutive operations. J Hand Surg Eur Vol. 2014;39(5):553-554.
16. Peimer CA, Wilbrand S, Gerber RA, et al. Safety and tolerability of collagenase Clostridium histolyticum and fasciectomy for Dupuytren’s contracture. J Hand Surg Eur Vol. 2015;40(2):141-140.
17. Armstrong JR, Hurren JS, Logan AM. Dermofasciectomy in the management of Dupuytren’s disease. J Bone Joint Surg Br. 2000;82(1):90-94.
To comment on this article, contact rdavidson@uspharmacist.com.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.






