US Pharm. 2007;32(2):HS17-HS23.
Angiotensin receptor blockers (ARBs) and
angiotensin-converting enzyme (ACE) inhibitors are highly utilized classes of
medications that affect the renin-angiotensin-aldosterone system (RAAS). Both
have been shown to be beneficial in the treatment of disease states--such as
hypertension, chronic heart failure, chronic kidney disease, and myocardial
infarction--in which the RAAS system plays a significant role.1-4
ACE inhibitors and ARBs have similar pharmacologic actions, with the primary
effect being interference of angiotensin II via either receptor inhibition
(ARBs) or decreased production (ACE inhibitors). Angiotensin II is a potent
vasoconstrictor that increases sodium reabsorption, contributes to ventricular
hypertrophy, and produces reactive oxygen species.5 Due to their
similar pharmacologic action, ACE inhibitors and ARBs are thought to have
equivalent efficacy for many indications; however, significant differences
exist with regard to tolerability. Cough and angioedema occur significantly
less frequently with ARBs than they do with ACE inhibitors.
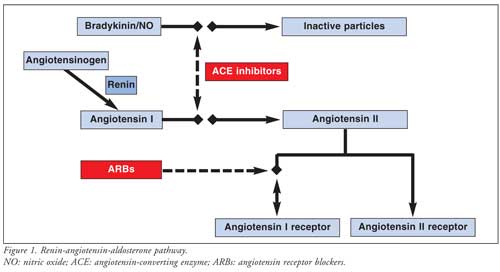
Up to 39% of patients taking ACE inhibitors experience cough,6-8 presumably related to increased levels of bradykinin, a potent vasoactive peptide inactivated by ACE (Figure 1). 5,9,10 Since ARBs do not affect ACE, the incidence of cough in patients taking these agents is much lower and has been found to be comparable to that of placebo in large clinical trials.11,12 It has become common practice to substitute ARBs for ACE inhibitors to alleviate cough.13 Controversy exists, however, with regard to caring for patients with ACE inhibitor–induced angioedema. Given the known mortality benefit of using ACE inhibitors and ARBs in both heart failure and post–myocardial infarction, identifying the incidence of ARB-induced angioedema, specifically the cross-reactivity of ARBs with ACE inhibitor–induced angioedema, carries important ramifications for clinical practice.
Angioedema
Angioedema is a localized, noninflammatory, nonpruritic swelling of the skin
characterized by a buildup of fluid in the interstitial tissue. It is often
benign but can result in asphyxiation when affecting the skin around the
larynx. It can also involve the lips, tongue, intestine, and other peripheral
tissues, particularly those containing less connective tissue. Genetic
disorders, such as C1 esterase inhibitor protein deficiency, and seasonal
allergies are also risk factors for the development of angioedema.14,15
The common cellular mechanism among these causes appears to be activation of
the complement system and/or other pro-inflammatory cytokines such as
prostaglandins and histamine, which can result in rapid vasodilatation and
edema.16 In addition to ARBs and ACE inhibitors, multiple
medications, including aspirin and nonsteroidal anti-inflammatory medications,
can also induce angioedema.
ACE inhibitor–induced angioedema accounts for 17% of all patients admitted to medical centers for the treatment of angioedema. 17,18 Studies report that anywhere from 13% to 22% of patients with ACE inhibitor–induced angioedema will require airway intervention. It has been theorized that ACE inhibitor–mediated angioedema results from an elevation in bradykinin levels, similar to cough.9 As in other causes of angioedema, elevated bradykinin levels in the peripheral tissues results in rapid fluid accumulation. Currently, this is the most accepted theory for why ACE inhibitors cause angioedema, but it has not been definitively proven. It also offers a scientifically sound hypothesis for why ARBs are less likely to cause angioedema and might serve as potential alternatives in patients with ACE inhibitor–induced angioedema.19,20 Instances of ARB-induced angioedema without ACE inhibitor exposure have also been described. Since ARBs do not increase bradykinin levels to the same degree as ACE inhibitors, the mechanism by which angioedema occurs with these medications is largely unknown. One theory is that stimulation of the angiotensin II receptor activates the bradykinin-prostaglandin-nitric oxide cascade, resulting in bradykinin-mediated side effects from both ARBs and ACE inhibitors; however, the angiotensin II function has not been fully elucidated. 19 Thus, until the exact mechanism of angioedema induced by these medications is known, patients must be monitored for symptoms associated with either ACE inhibitors or ARBs. Given the potential life-threatening nature of angioedema, a review of case reports, case series, clinical trial data, and scientific literature will help determine if hesitancy over cross-reactivity is justified.
Incidence of ACE Inhibitor– and ARB-Induced
Angioedema
The first case report of ACE inhibitor–induced angioedema occurred in 1980,
and now, 2 to 10 per 1,000 patients reportedly experience this adverse effect.
15,21 More than 40 million people receive ACE inhibitors worldwide, 60%
of whom develop ACE inhibitor–induced angioedema during the first week of
medication administration.16 This early initiation period has also
been associated with a 14-fold increase in the incidence of angioedema.
9,16,21 The elevated risk persists during the first month of therapy,
accounting for a ninefold increase in incidence.16 There are,
however, case reports of angioedema beyond one year of therapy, possibly
resulting from nonadherence or dose escalation.16 The risk of
developing angioedema has also been associated with smoking, advanced age,
being female or black, and having seasonal allergies.21,22
The exact prevalence and incidence of ARB-induced angioedema are not known, but are thought to be significantly lower than those of ACE inhibitors.17 In fact, in 1995, when the first ARBs were approved for the treatment of hypertension, the risk of angioedema was considered negligible;23 however, postmarketing surveillance has shown that this reaction can occur with ARBs. The majority of cases of ARB-mediated angioedema have been associated with administration of losartan. It is unclear at this time, however, if all ARBs within the class affect bradykinin to the same extent, or if another mechanism exists.10 In one study, Johnsen and colleagues searched a hospital discharge registry of more than 1.4 million individuals for the incidence of first hospitalization for nonhereditary angioedema.17 Using prescription databases, they identified 641 cases of angioedema and cross-referenced these patients to determine the number of individuals who were currently receiving or had been receiving ACE inhibitors or ARBs. These cases were then matched with 6,264 control patients from the general population. The incidence of angioedema was highest in patients who were currently receiving or had received an ACE inhibitor. Past or present ARB use was not associated with an increase in first-time hospitalization for angioedema in this study. Although the risk of angioedema might be lower with ARBs as initial therapy, these findings do not confirm whether ARBs are appropriate in a patient who has already experienced ACE inhibitor–induced angioedema. The remainder of this paper will focus on the literature surrounding this particular question.
Literature Review of Cross-Reactivity
The issue of ARB and ACE inhibitor cross-reactivity has been addressed in
several case reviews. A MEDLINE search conducted by Warner and colleagues
reported findings from one case series and six case reports between 1966 and
1999 that addressed angio edema in patients previously experiencing this
adverse reaction with an ACE inhibitor.21 The case series in their
review included 13 patients with losartan-induced angioedema. Three of the
patients had experienced reactions to an ACE inhibitor.14 Since the
publication of this review, other case reports have echoed the same concern of
cross-reactivity. Cha and Pearson described a 62-year-old black woman who was
admitted for the management of hypertension.13 The patient was
initially treated with beta-blockade and ACE inhibition, and developed
angioedema four days after initiation of captopril. Losartan was then
substituted for captopril, and the patient developed a similar swelling after
only one dose. Likewise, in 2002, a case report described a 27-year-old female
patient with type 1 diabetes who was being treated with enalapril for
microalbuminuria.7 She developed angioedema, which resolved with
discontinuation of enalapril. At her follow-up two months later, she was
prescribed losartan. After three days, she developed a swollen throat and
tongue and was diagnosed again with angioedema. These articles and others have
prompted the initiation of several clinical trials to investigate if an ARB
can be used as a therapeutic alternative in ACE inhibitor–intolerant patients,
including those with angioedema.
Case reports demonstrate that there is some degree of angioedema cross-reactivity between ACE inhibitors and ARBs; however, the reports fail to describe the incidence of or identify the mechanism for this reaction. A trial of 54 patients, all of whom experienced ACE inhibitor–induced angioedema, was performed to determine the safety of using other antihypertensive medications in this population.24 After discontinuing the offending agent, 26 patients were switched to an ARB, 14 to a calcium channel blocker, and 14 to other various antihypertensive medications. The patients were followed for one to 80 months, and 81% of the patients who received an ARB had complete resolution or reduction of angioedema, compared with 78% of those receiving calcium channel blockers. The authors reported recurrence of angioedema in only two of 26 patients (8%) receiving ARBs. A similarly low cross-reactivity was demonstrated in the angioedema subgroup of patients enrolled in the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity–Alternative trial (CHARM-Alternative), in which 2,028 patients with systolic heart failure who were ACE inhibitor–intolerant were randomized to receive candesartan or matching placebo.25 Of the 83 patients who had previously experienced ACE inhibitor–induced angioedema or anaphylaxis, 39 received candesartan. During the 38 months of follow-up, angioedema recurred in three patients (7.7%), and only one patient (2.6%) required discontinuation of candesartan. On the basis of these two trials, cross-reactivity appears to be less than 10%, but given the frequency of treatment with these two classes of medications, further data are needed to validate these results. Currently, the Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease (TRANSCEND) trial, another trial of ARBs in patients who are ACE inhibitor–intolerant, is underway.26 The TRANSCEND trial is recruiting patients from 730 centers and 40 countries and is projected to enroll more than 6,000 subjects who will receive either telmisartan or placebo. The study's primary outcome is the composite end point of cardiovascular death, myocardial infarction, stroke, or hospitalization for heart failure. Depending on the number of patients recruited who have a history of ACE inhibitor–induced angioedema, the trial might provide more conclusive data on the incidence of cross-reactivity between ACE inhibitors and ARBs.
Discussion
A major limitation of postmarketing surveillance and adverse event reporting
is the lack of data on successful actions in clinical practice. There are
multiple case reports, case series, and adverse event data to suggest using
caution when initiating ARBs in patients who previously developed angioedema
with ACE inhibitor therapy. It is not known, however, how many times this
substitution is performed successfully and without adverse effect to the
patient in day-to-day clinical practice. We do know that ARBs and ACE
inhibitors have demonstrated a reduction in mortality for several patient
populations, including those with heart failure and those who have experienced
myocardial infarction. We also know that these classes of medications have
been shown to slow progression of diabetic nephropathy and chronic kidney
disease. To date, the best data on using ARBs in patients with ACE
inhibitor–induced angioedema have come from the prospective, randomized
CHARM-Alternative trial, with cross-reactivity angioedema resulting in
discontinuation of therapy in less then 3% of patients.25 Since
recurrence of angioedema was not a primary outcome, and only 4% of the patient
population had previous angioedema, results of the CHARM-Alternative trial
cannot be considered definitive. It is hoped that the TRANSCEND trial will
corroborate these results with an even larger patient population and further
substantiate the role of ARBs in ACE inhibitor–intolerant patients.
Further study is needed on the mechanism for angioedema jointly induced by these agents. Pharmacologically, it seems an ARB should be safe to use in a patient with ACE inhibitor–induced angioedema; however, we know from the case reports and literature review that cross-reactivity does occur, albeit at a relatively low frequency. It is possible that there are common pathways for ACE inhibitor– and ARB-induced angioedema beyond bradykinin that have yet to be discovered.
Based on the relatively low prevalence of cross-reactivity in the literature (<10%), and the benefits of angiotensin II inhibition for certain disease states, ARBs should be considered in patients with ACE inhibitor–induced angioedema. Thus far, this stance has been adopted by the National Kidney Foundation guidelines and the American College of Cardiology and American Heart Association (ACC/AHA) consensus guidelines for the treatment of heart failure, acute myocardial infarction, and the secondary prevention of cardiovascular disease.27-30 Given the strong potential for harm with drug-induced angioedema, however, close monitoring is necessary to ensure that repeat angioedema does not occur. As new information regarding the mechanism of drug-induced angioede ma or the cross-reactivity of ACE inhibitor–induced angioedema with ARBs becomes available, this issue will require further scrutiny. For now, populations that have demonstrated a clear benefit from angiotensin II antagonism who have no alternative--such as those with heart failure or chronic kidney disease, or those who have had a myocardial infarction--require the best efforts of the practitioner to initiate these life-prolonging therapies.
References
1. Remuzzi G, Ruggenenti P. Overview of randomised trials of ACE inhibitors.
Lancet. 2006;368:555-556.
2. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the
angiotensin-receptor antagonist irbesartan in patients with nephropathy due to
type 2 diabetes. N Engl J Med. 2001;345:851-860.
3. Casas JP, Chua W, Loukogeorgakis S, et al. Effect of inhibitors of the
renin-angiotensin system and other antihypertensive drugs on renal outcomes:
systematic review and meta-analysis. Lancet. 2005;366:2026-2033.
4. Sleight P. Angiotensin II and trials of cardiovascular outcomes. Am J
Cardiol. 2002;89:11A-16A.
5. Dzau V. The cardiovascular continuum and renin-angiotensin-aldosterone
system blockade. J Hypertens Suppl. 2005;23:S9-S17.
6. Israili ZH, Hall W. Cough and angioneurotic edema associated with
angiotensin-converting enzyme inhibitor therapy. A review of the literature
and pathophysiology. Ann Intern Med. 1992;117:234-242.
7. Abdi R, Dong VM, Lee CJ, et al. Angiotensin II receptor blocker-associated
angioedema: on the heels of ACE inhibitor angioedema. Pharmacotherapy.
2002;22:1173-1175.
8. Tolerability and quality of life in ARB-treated patients. Am J Manag Care
. 2005;11:S392-S394.
9. Vleeming W, Van Amsterdam JG, Stricker BH, de Wilt DJ. ACE
inhibitor-induced angioedema. Incidence, prevention, and management. Drug
Saf. 1998;18:171-188.
10. Chiu AG, Krowiak EJ, Deeb ZE. Angioedema associated with angiotensin II
receptor antagonists: challenging our knowledge of angioedema and its
etiology. Laryngoscope. 2001;111:1729-1731.
11. Goldberg AI, Dunlay MC, Sweet CS. Safety and tolerability of losartan
potassium, an angiotensin II receptor antagonist, compared with
hydrochlorothiazide, atenolol, felodipine ER, and angiotensin-converting
enzyme inhibitors for the treatment of systemic hypertension. Am J Cardiol
. 1995;75:793-795.
12. Lacourciere Y, Brunner H, Irwin R, et al. Effects of modulators of the
renin-angiotensin-aldosterone system on cough. Losartan Cough Study Group.
J Hypertens. 1994;12:1387-1393.
13. Cha YJ, Pearson VE. Angioedema due to losartan. Ann Pharmacother.
1999;33:936-938.
14. van Rijnsoever EW, Kwee-Zuiderwijk WJ, Feenstra J. Angioneurotic edema
attributed to the use of losartan. Arch Intern Med. 1998;158:2063-2065.
15. Sarkar P, Nicholson G, Hall G. Brief review: angiotensin converting enzyme
inhibitors and angioedema: anesthetic implications. Can J Anaesth.
2006;53:994-1003.
16. Byrd JB, Adam A, Brown NJ. Angiotensin-converting enzyme
inhibitor-associated angioedema. Immunol Allergy Clin North Am.
2006;26:725-737.
17. Johnsen SP, Jacobsen J, Monster TB, et al. Risk of first-time
hospitalization for angioedema among users of ACE inhibitors and angiotensin
receptor antagonists. Am J Med. 2005;118:1428-1429.
18. Agostoni A, Cicardi M. Drug-induced angioedema without urticaria. Drug
Saf. 2001;24:599-606.
19. Fuchs SA, Meyboom RH, van Puijenbroek EP, Guchelaar HJ. Use of angiotensin
receptor antagonists in patients with ACE inhibitor-induced angioedema.
Pharm World Sci. 2004;26:191-192.
20. Irons BK, Kumar A. Valsartan-induced angioedema. Ann Pharmacother.
2003;37:1024-1027.
21. Warner KK, Visconti JA, Tschampel MM. Angiotensin II receptor blockers in
patients with ACE inhibitor-induced angioedema. Ann Pharmacother. 2000;
34:526-528.
22. Kostis JB, Packer M, Black HR, et al. Omapatrilat and enalapril in
patients with hypertension: the Omapatrilat Cardiovascular Treatment vs.
Enalapril (OCTAVE) trial. Am J Hypertens. 2004;17:103-111.
23. Goodfriend TL, Elliott ME, Catt KJ. Angiotensin receptors and their
antagonists. N Engl J Med. 1996;334:1649-1654.
24. Cicardi M, Zingale LC, Bergamaschini L, et al. Angioedema associated with
angiotensin-converting enzyme inhibitor use: outcome after switching to a
different treatment. Arch Intern Med. 2004;164:910-913.
25. Granger CB, McMurray JJ, Yusuf S, et al. Effects of candesartan in
patients with chronic heart failure and reduced left-ventricular systolic
function intolerant to angiotensin-converting-enzyme inhibitors: the
CHARM-Alternative trial. Lancet. 2003;362:772-776.
26. Unger T. The ongoing telmisartan alone and in combination with ramipril
global endpoint trial program. Am J Cardiol. 2003;91:28G-34G.
27. Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the
Diagnosis and Management of Chronic Heart Failure in the Adult: a report of
the American College of Cardiology/American Heart Association Task Force on
Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the
Evaluation and Management of Heart Failure): developed in collaboration with
the American College of Chest Physicians and the International Society for
Heart and Lung Transplantation: endorsed by the Heart Rhythm Society.
Circulation. 2005;112:e154-e235.
28. Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the
management of patients with ST-elevation myocardial infarction: a report of
the American College of Cardiology/American Heart Association Task Force on
Practice Guidelines (Committee to Revise the 1999 Guidelines for the
Management of Patients with AcuteMyocardial Infarction). Circulation.
2004;110:e82-e292.
29. Smith SC Jr, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary
prevention for patients with coronary and other atherosclerotic vascular
disease: 2006 update endorsed by the National Heart, Lung, and Blood
Institute. J Am Coll Cardiol. 2006;47:2130-2139.
30. AHA; ACC; National Heart, Lung, and Blood Institute; Kidney Disease
Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on
hypertension and antihypertensive agents in chronic kidney disease. Am J
Kidney Dis. 2004;43(5 suppl 1):S1-S290.
To comment on this article, contact editor@uspharmacist.com.





