US Pharm. 2019;44(4):16-19.
ABSTRACT: Statin medications (hydroxymethylglutaryl-coenzyme A reductase inhibitors) have been widely used for their efficacy in lowering cholesterol and reducing the risk of cardiovascular disease. Studies have shown that patients who take statins may have a reduced immune response to the influenza vaccine compared with patients who do not take statins. Research has shown that patients taking statins who receive the influenza vaccine may be at higher risk for contracting influenza compared with those not taking statins who receive the vaccine. Further studies may be warranted to determine other confounders or potential ways to avoid this problem.
Statin medications, or hydroxymethylglutaryl-coenzyme A reductase inhibitors, have been widely used for their efficacy in lowering cholesterol and reducing the risk of cardiovascular disease in at-risk patients. Notably, statins also have profound immunomodulatory anti-inflammatory effects.1 For example, it has been shown that among the unvaccinated population patients who take statins have a lower mortality risk than patients not taking statins.2 It has also been noted that statins suppress T-cell activation.1
Statin Indications
In 2017, nearly 20% of the U.S. population older than 40 years and about 50% of the U.S. population older than 75 years were using a statin medication.3 Statins are used for primary and secondary prevention of cardiovascular disease in at-risk patients. According to the 2018 American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, patients have an indication for statin therapy if they meet any of following criteria4:
• Patient is younger than 75 years and has a history of atherosclerotic cardiovascular disease.
• Patient has an LDL cholesterol of >190 mg/dL.
• Patient is aged between 40 and 75 years, has diabetes, and has an LDL of >70 mg/dL.
• Patient is aged between 40 and 75 years and has a 10-year atherosclerotic cardiovascular disease risk of >7.5%.
Atherosclerotic cardiovascular disease is defined as the presence of a history of myocardial infarction, acute coronary syndrome, stable or unstable angina, coronary or arterial revascularization, stroke, transient ischemic attack, or peripheral arterial disease of presumed atherosclerotic origin.4 A patient’s 10-year risk of atherosclerotic cardiovascular disease may be calculated by using the ASCVD Risk Estimator Plus (available through the American College of Cardiology), which takes into consideration information such as the patient’s cholesterol, blood pressure, smoking history, diabetes, sex, and race.5 The ASCVD Risk Estimator Plus is intended for use in primary-prevention patients.
Influenza Virus
The influenza virus occurs in a cycle that begins with an interaction between hemagglutinin glycoprotein and sialic acid residues on the host-cell proteins. During the cycle, hemagglutinin, neuraminidase, and matrix 2 (M2) ion-channel proteins are expressed on the surface of the host cell. The hemagglutinin glycoprotein is most dominantly expressed, followed by the neuraminidase glycoprotein.6 It has been shown that influenza viruses induce many biological mediators of inflammation, including several proinflammatory cytokines.6 Cytokine dysregulation has also been seen in specific strains of influenza, including H5N1 and the 1918 pandemic influenza virus.2 Hemagglutinin, neuraminidase, and M2 have been evaluated as vaccine targets.
Influenza Vaccine
The influenza virus is associated with increased titers of serum antibodies directed at the viral hemagglutinin. The influenza vaccine is formulated to mimic the body’s response to the influenza virus. In 2013, the FDA approved an influenza vaccine that is produced without propagation of the influenza virus. This recombinant hemagglutinin–based vaccine is able to mimic the antihemagglutinin immune response of the vaccine that had been produced by the inactivated influenza virus. Prior to 2013, the vaccine contained inactivated whole viruses that were delivered in subunit or split preparations. Despite differences in preparation, the newer vaccine has the ability to induce hemagglutinin-specific antibodies.6
Influenza-Vaccine Indications
The influenza vaccine is indicated for the prevention of influenza caused by both A subtype viruses and B subtype viruses in patients aged 6 months and older. The influenza vaccine should not be administered to patients with a history of allergic reaction to any component of the vaccine or to a previous influenza vaccine. Patients who are immunocompromised or who are receiving immunosuppressive therapy may not achieve the expected immune response.7
Studies on Statin Use and Influenza-Vaccine Response
A PubMed search was conducted to identify available literature on statin use and influenza-vaccine effectiveness. Search criteria included the MeSH terms hydroxymethylglutaryl-coenzyme A reductase inhibitors, pharmacologic actions, statin, influenza vaccines, and flu vaccine. The references were limited to the English language and individually reviewed for relevance. Results of the studies are summarized in TABLE 1.
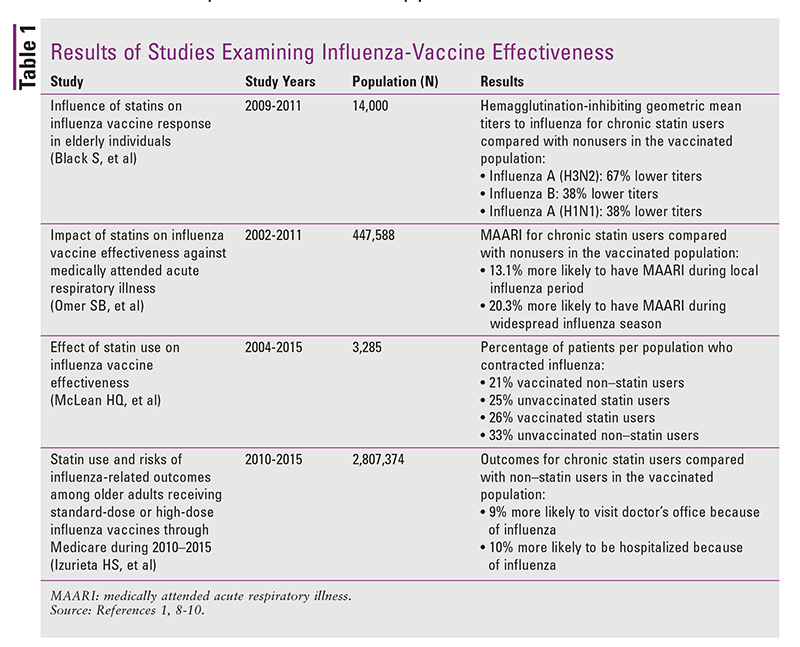
For the 2009-2010 influenza season and then again for the 2010-2011 season, 14,000 patients older than 65 years participated in a randomized, controlled, observer-blind clinical trial investigating the efficacy of the influenza vaccine in elderly persons given statin therapy.1 The trial was conducted in the U.S., Colombia, Panama, and the Philippines. Patients were considered to be receiving statin therapy if they had been taking a statin 28 days prior to the vaccine as well as 22 days afterward. The statin-therapy cohort demonstrated consistently lower hemagglutination-inhibiting geometric mean titers to influenza. Patients receiving statin therapy had 38% lower titers (95% CI, 27%-50%) for the H1N1 variety of influenza A, 67% lower titers (95% CI, 54%-80%) for the H3N2 variety of influenza A, and 38% lower titers (95% CI, 28%-29%) for influenza B. Although this study indicated a likely immunosuppressant effect on vaccine immune response, it was unable to determine the clinical impact of the reduction in titers. In a secondary outcome, this study also found that patients taking synthetic statins (fluvastatin, atorvastatin, and rosuvastatin) had more pronounced immunosuppression compared with patients taking fermentation-derived statins (pravastatin, simvastatin, lovastatin, and Advicor [niacin/lovastatin]).1
A 9-year U.S. retrospective cohort study conducted in Georgia from 2002 to 2011 also found that vaccine effectiveness may be limited by statin therapy. Patient information was obtained from Kaiser Permanente managed-care organization records. This study measured medically attended acute respiratory illness (MAARI) as its primary outcome, rather than incidence of diagnosed influenza. Patients receiving statin therapy were found to be 13.1% (95% CI, 0.4%-27.6%) more likely to have MAARI during times of high incidence of influenza locally and 20.3% (95% CI, 5.1%-37.6%) more likely to have MAARI during times of widespread influenza compared with patients not receiving statin therapy. Although these results appear to show reduced efficacy of the influenza vaccine in patients receiving statin therapy, it must be taken into consideration that MAARI encompasses not just influenza but also strep throat, pneumonia, and other respiratory illnesses.8
From 2004 to 2015, Marshfield Clinic in Marshfield, Wisconsin, enrolled 3,957 patients older than 45 years to evaluate the relationship between statin therapy and decreased efficacy of the influenza vaccine. Patients were recruited from outpatient and urgent-care settings where they had presented with an acute respiratory illness. Patients were considered to be receiving statin therapy if they had used a statin in the past 2 years and had received the influenza vaccine at least 14 days prior to seeking medical attention. It was determined that 26% of vaccinated statin users contracted influenza A, 25% of unvaccinated statin users contracted influenza, 21% of vaccinated nonusers had influenza, and 33% of unvaccinated nonusers had influenza. Ultimately, it was determined that patients who were given the influenza vaccine and were receiving statin therapy were at higher risk for influenza than those who did not take statin medications, but both groups had a lower risk of influenza than persons who did not receive an influenza vaccination.9
A retrospective cohort study based on Medicare data examined patients older than 65 years who were enrolled in Medicare Parts A, B, and D and received an influenza immunization from a community pharmacy in the influenza seasons from 2010 to 2015. The primary outcome was doctor’s office visits and hospital visits in which there was a positive rapid influenza diagnostic test result. The relative risk (RR) was significant for office visits (RR, 1.09; 95% CI, 1.03-1.15) and for influenza hospitalizations (RR, 1.10; 95% CI, 1.02-1.19), demonstrating an approximate 10% increase in risk of influenza in patients taking statins who received an influenza immunization versus patients not taking statins who received an influenza immunization.10
Need for Further Study
Although it has been shown that patients who receive the influenza vaccine and are concurrently taking statin medications may be at greater risk for influenza than patients not taking statins who receive the vaccine, further studies may be warranted to determine other confounders or potential ways to avoid this problem. Even though the microneedle patch provides some efficacy, it is possible that other dosage forms may be more beneficial for patients taking statins. Also to be considered is whether the high-dose influenza vaccine that is typically reserved for immunocompromised persons or those older than 65 years would impact the efficacy of the influenza vaccine in patients who take statins. Another factor that may be worth investigating is the effect of statin therapy on other common vaccines, such as those for pneumonia and shingles.
Based on published data that statin therapy reduces the efficacy of the influenza vaccine, studies have begun to examine an alternative method of influenza protection. A 2017 study in mice given statins and those not given statins compared hemagglutinin-inhibition titers in mice that were exposed to the influenza vaccine either systemically or via a dissolving microneedle patch.3 It was found that compared with systemically treated mice, mice that received the vaccine via the microneedle patch had higher hemagglutinin-inhibition titers and roughly 20 times the total antibody level to the influenza vaccine.3
Conclusion
Studies have indicated that patients receiving statin therapy have a reduced immune response to the influenza vaccine compared with patients not taking statins; however, data are insufficient to support withholding influenza vaccine in a patient receiving statin therapy. In addition, information is lacking to support withholding statin therapy in patients in whom the seasonal influenza immunization is indicated. The studies evaluated demonstrated that patients who were taking statins and received the influenza vaccine had a lower risk of contracting the influenza virus compared with patients who were not vaccinated. In addition, statin therapy was shown to reduce the incidence of influenza in patients who did not receive the influenza vaccine.2 Overall, the benefit of statin therapy for preventing cardiovascular disease outweighs the risk of a reduced influenza-vaccine response, and the benefit of vaccination outweighs the risk of a reduced response in patients receiving statin therapy.
REFERENCES
1. Black S, Nicolay U, Del Giudice G, Rappuoli R. Influence of statins on influenza vaccine response in elderly individuals. J Infect Dis. 2015;213(8):1224-1228.
2. Vandermeer ML, Thomas AR, Kamimoto L, et al. Association between use of statins and mortality among patients hospitalized with laboratory-confirmed influenza virus infections: a multistate study. J Infect Dis. 2012;205(1):13-19.
3. Vassilieva EV, Wang S, Li S, et al. Skin immunization by microneedle patch overcomes statin-induced suppression of immune responses to influenza vaccine. Sci Rep. 2017;7(1):17855.
4. Grundy S, Stone N, Bailey A, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018 November 8 [Epub ahead of print].
5. American College of Cardiology. ASCVD Risk Estimator Plus. http://tools.acc.org/ASCVD-Risk-Estimator-Plus/#!/calculate/estimate. Accessed September 18, 2018.
6. Huber VC. Influenza vaccines: from whole virus preparations to recombinant protein technology. Expert Rev Vaccines. 2014;13(1):31-42.
7. Fluarix (influenza vaccine) package insert. Research Triangle Park, NC: GlaxoSmithKline; July 2010.
8. Omer SB, Phadke VK, Bednarczyk RA, et al. Impact of statins on influenza vaccine effectiveness against medically attended acute respiratory illness. J Infect Dis. 2015;213(8):1216-1223.
9. McLean HQ, Chow BD, VanWormer JJ, et al. Effect of statin use on influenza vaccine effectiveness. J Infect Dis. 2016;214(8):1150-1158.
10. Izurieta HS, Chillarige Y, Kelman JA, et al. Statin use and risks of influenza-related outcomes among older adults receiving standard-dose or high-dose influenza vaccines through Medicare during 2010–2015. Clin Infect Dis. 2018;67(3):378-387.
To comment on this article, contact rdavidson@uspharmacist.com.





