US Pharm. 2023;48(1):HS-2-HS-9.
ABSTRACT: Neuromyelitis optica (NMO) is an autoimmune disorder of the central nervous system that targets the optic nerves and the spinal cord, resulting in optic neuritis and myelitis, respectively. Neuromyelitis optica spectrum disorder has estimated worldwide prevalence and average annual incidence of 1-5/100,000 and 1/770,000, respectively. Due to patterns of relapses and remission, NMO may be difficult to distinguish from multiple sclerosis. NMO more commonly affects females, comprising about 90% of cases, and typically occurs more in black and Eastern Asian persons in Western countries. Symptoms may consist of pain, bowel and bladder dysfunction, fatigue, cognitive impairment, and psychiatric disturbances. Treatment consists of medication management for acute relapses and symptomatic and maintenance therapy for relapse prevention. There are currently only three drugs that are FDA approved for NMO.
Neuromyelitis optica (NMO) is a chronic, demyelinating inflammatory autoimmune disorder of the central nervous system (CNS) affecting both the spinal cord (myelitis) and optic nerves (optic neuritis). NMO was first described by Eugene Devic and his student Fernand Gault, thus NMO is also referred to as Devic’s disease.1-3 With better understanding of the disease, its detection, and the most recent diagnostic criteria, a new broader term has been coined as neuromyelitis optica spectrum disorder (NMOSD), which includes more restrictive forms, such as recurrent optic neuritis and relapsing transverse myelitis.3,4 NMO/NMOSD results in recurrent attacks that can lead to severe disability, blindness, and paralysis.5,6 Symptoms can be grouped as visual (blindness), motor (spasms/spasticity/paralysis) and/or sensory dysfunction (neuropathic pain, bowel and bladder dysfunction, and sexual dysfunction), and psychiatric or cognitive impairment.7
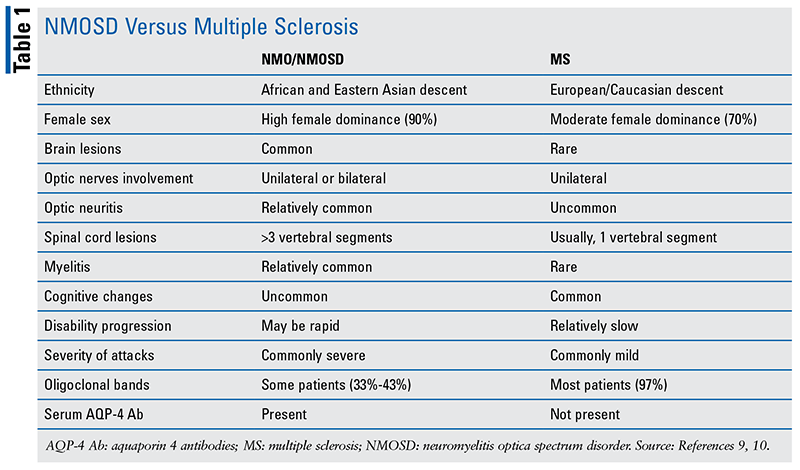
The incidence and prevalence of NMO may vary according to location, sex, and ethnicity. NMO/NMOSD more commonly occurs in women compared with men, at a 9:1 ratio, with an average age of onset of about 40 years.2,8,9 Individuals of African ethnicity have the highest prevalence and incidence compared to persons of white ethnicity.10 In addition, African Americans and Asians typically have a lower average age of onset compared with whites. Individuals of African descent have a higher risk of disability and mortality rate compared with other ethnicities.1,11 NMOSD has estimated worldwide prevalence and average annual incidence of 1-5/100,000 and 1/770,000, respectively.12 Since NMOSD causes severe disability with periods of attacks or relapses, hospital, doctor’s office visits, and pharmacy costs are substantial. The average all-cause healthcare cost among patients with NMO/NMOSD was $60,599 per year, with an annual cost of relapse of $10,070 per patient.13 It therefore is the hope that medications that can prevent relapses will decrease hospitalizations, outpatient visits, and treatment cost. Although NMO/NMOSD can be found in children, the scope of this article will be the diagnosis and management in adults.
Etiology and Pathophysiology
Due to its similarities to multiple sclerosis (MS), identification of pathogenic autoantibodies became an important milestone in NMO/NMOSD, and the disease is no longer considered a subtype or variant to MS.3,14 (See TABLE 1.) To gain insight into the pathogenesis of NMO/NMOSD, a proper understanding of some key roles and responsibilities of neurons, astrocytes, the blood-brain barrier (BBB), aquaporin 4 (AQP-4), lymphocytes, aquaporin 4 antibodies (AQP-4 Abs), the complement system, and interleukins (IL) are imperative. In addition, myelin oligodendrocyte glycoprotein (MOG) has become a target in the pathogenesis of NMO.14 Neurons or nerve cells are responsible for the motor commands to muscles and for transmitting electrical impulses.15
Astrocytes help develop and keep neurons in place, create synapses, stimulate nervous tissue repair, and help with the formation and maintenance of the BBB.16,17 The BBB is the barrier between brain blood vessels and brain tissue, blocking the passage of bacteria, viruses, and immune cells from entering the CNS, preventing brain damage.18,19 AQP-4 is a water protein that is responsible for brain water transport, cell migration, and cell homeostasis found in the optic nerve, brain, and spinal cord. AQP-4 is localized to the foot processes of astrocytes bordering the BBB and contributes to water transport across the BBB.1,20 Lymphocytes are a type of white blood cell. The primary forms of lymphocytes are B-lymphocytes (B-cells) and T-lymphocytes (T-cells). B-cells are responsible for antibody production, while T-cells kill tumor cells and help control immune responses.21,22
B-cells express cluster differentiation (CD) antigen receptors on their cell surface. The binding of foreign or toxin substances, known as antigens, to CD antigen receptors leads to B-cell activation. Upon B-cell activation, plasmablasts are formed.23 Plasmablasts are immature blood cells of a plasma cell that can secrete antibodies, such as AQP-4 Abs.24 The binding of AQP-4 Abs, specifically the immunoglobulin G (IgG) subtype to AQP-4 receptors in astrocytes, can lead to reduced BBB function, allowing the AQP-4 Abs to cross the BBB, resulting in disease.1-3,14 In addition, the binding of AQP-4 Abs to AQP-4 receptors can lead to activation of complement cascade and cause astrocytic damage. In some conditions, complement-dependent changes can promote nerve regeneration, tissue repair, and healing. However, in other conditions, dysregulation of the complement cascade leads to chronic inflammation, persistent pain, and neural dysfunction.
There are three complement system pathways: classical, alternative, and lectin. All three pathways converge to produce the common complex C3 convertase, cleaving the C3 component of the system into C3a and C3b. C3b can help form C5 convertase, which ultimately cleaves C5 into C5a and C5b. C5b leads to the membrane attack complex (MAC), and C5a leads to anaphylatoxin, with both MAC and anaphylatoxin causing cell injury and production of proinflammatory mediators, respectively.3,4,8,14,25 In response to inflammation, ILs, a type of cytokine, are secreted from leukocytes (white blood cells including lymphocytes). IL-6 is involved in plasmablast survival and disruption of the BBB, among other functions.1,8,25
In summary, B-cell activation occurs through antigen binding to the CD antigen receptors. B-cells, through various mechanisms, produce antibodies such as AQP-4 Abs. These antibodies can lead to IL production and the induction of the complement cascade ultimately causing astrocytic damage. Once the astrocyte is damaged, it can no longer support the BBB and neurons, thereby leading to demyelination of the neuron and BBB damage. Therefore, inhibition of the B-cell production, complement pathway induction, and/or IL secretion are important ways of preventing the astrocytic damage and BBB dysfunction. Another mechanism to the pathophysiology of NMOSD is the MOG, which is found on oligodendrocyte of the myelin sheath. The myelin sheath helps protect nerve cells. MOG antibodies (MOG-Abs) can damage the myelin (demyelination), thereby preventing electrical impulses from transmitting quickly and efficiently.1,2
Diagnosis and Clinical Manifestations
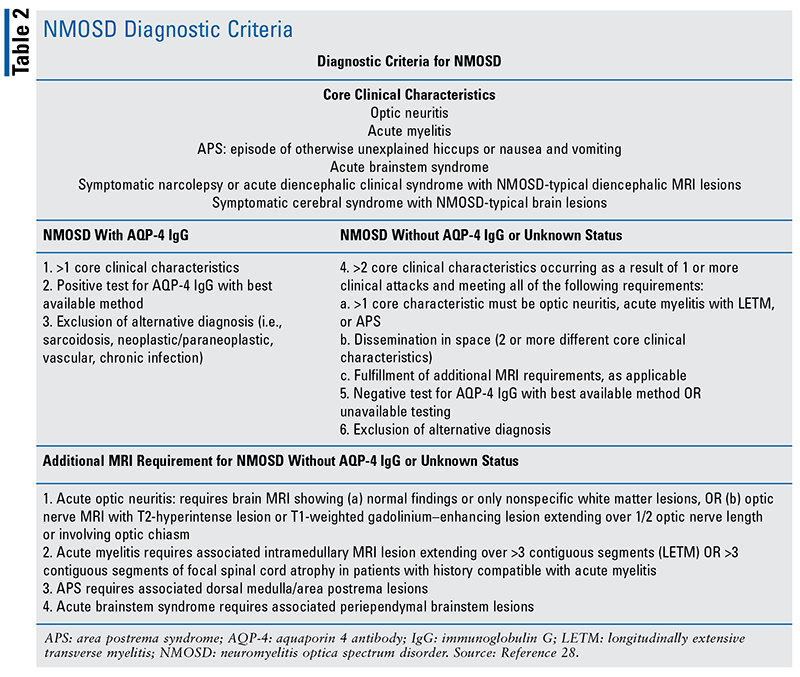
The understanding of NMO/NMOSD has evolved throughout the years. It was thought that it was a variant of MS, which can mimic NMO/NMOSD, but with the identification of AQP-4, it is noted to be its own distinct disease and can be used to help distinguish it from classical MS.4,26,27 In addition, the name and diagnosis have evolved since the first criteria in 1999 and its revision in 2006 to now the latest criteria established in 2015. In 2007, the term NMOSD was coined to include the AQP-4 seropositivity.14 Prior NMO diagnostic criteria required optic nerve and spinal cord involvement without brain manifestations or disease. To account for more restricted or more extensive CNS involvement, the new criteria not only included AQP-4 positivity but also now brain syndromes. The broader term of NMOSD can include NMO along with other forms, such as recurrent optic neuritis, transverse myelitis, and some encephalitic presentations.3,4,14 Now NMOSD can be divided into the following types: NMOSD with AQP4-IgG (NMOSD-AQP4) and NMOSD without AQP4-IgG or with unknown AQP4-IgG status.4 These latest diagnosic criteria are from the International Panel for NMO Diagnosis (see TABLE 2).28 These updated criteria have helped in early detection and improvement in disease outcomes. Seropositivity with AQP-4 Abs accounts for up to 90% of NMO cases. MOG-Abs account for approximately 42% of NMOSD patients who are seronegative for AQP-4.1
There are many inflammatory, neoplastic, and infectious diseases that can involve the CNS and mimic NMO/NMOSD.9 However, the AQP-4 Ab is a disease-specific autoantibody to NMO/NMOSD and is rarely found in other neurologic conditions. As a part of the diagnosic criteria, determining AQP-4 Abs status is needed when available and utilization of appropriate assay are essential. Testing of AQP-4 Abs can predict long-term prognosis as well as therapeutic response. Indirect immunofluorescence (IIF), enzyme-linked immunosorbent assay (ELISA), cell-based assay (CBA), and flow cytometry assay (FACS-assay) are used for antibody level detection.
CBAs are strongly recommended based on the 2015 international consensus diagnostic criteria. The IIF was the first assay to identify NMO-IgG and can be useful as a screening tool for diverse antibodies at a relatively low cost. The ELISA has a relatively low accuracy compared with the others. There are two types of CBAs, live and prefixed. The live-CBA has higher accuracy compared with fixed-CBA but requires more expertise, which may limit its use compared with the more widely and easily used fixed-CBA. If one of the two CBAs results does not match the clinical or radiological signs and symptoms, it is recommended to retest the sample with the other. In some cases, the FACS-assay can yield higher sensitivity than CBAs and supply the fixed-CBA or even the live-CBA. The FACS-assay could also be advantageous in that it can yield quantitative results and a cutoff discriminator.1,9
Management
Treatment Overview
There are multiple approaches when managing or treating patients with NMO/NMOSD that may include symptomatic therapies, management of acute relapses, and long-term maintenance with immunosuppressive therapies. Symptomatic therapy involves treating any pain; fatigue; bowel/bladder, cognitive, or sexual dysfunction; and spasms. Acute management of relapses involves therapy with corticosteroids and plasma exchange (PLEX). However, approximately 90% of patients may eventually relapse and/or demonstrate permanent disability. Therefore, long-term management of symptoms involving immunotherapy is of utmost importance in managing NMO/NMOSD, considering its relapse-related disability, poor prognosis, and high risk of mortality in untreated patients.5,29 Recent studies suggest immediate initiation of long-term immunotherapy once the diagnosis of NMO/NMOSD is determined. There are three monoclonal antibody agents that recently earned FDA approval for management of NMO/NMOSD. Rituximab, an anti-CD20 agent approved in 1997, has the strongest evidence to support its use, while mycophenolate has comparable reductions in absolute relapse rate and Expanded Disability Status Scale (EDSS) scores.30
Symptomatic and Restorative
Some symptoms experienced in NMO/NMOSD are related to pain, stiffness, fatigue, bladder and bowel symptoms, sexual dysfunction, spasms, and neuropsychiatric conditions.7 Pain severely impacts quality of life, with pain having a prevalence of over 80% in NMO/NMOSD. The pain syndrome experienced can be neuropathic, nociceptive, and/or mixed pain syndromes. NMO/NMOSD–related pain can be chronic in nature as the disease progresses or can develop during acute relapses.31 Fatigue in NMO/NMOSD affects quality of life, depression, sleep quality, and pain intensity, which worsens with high fatigue severity.32 Lower urinary tract symptoms occur in about 80% of NMO/NMOSD cases in which they may experience frequency, urgent and stress incontinence, nocturia, urinary retention, and incomplete evacuation. The most common lower urinary tract symptom is urinary retention.33 Like many other neurologic disorders, sexual dysfunction is common. Sexual dysfunction is common in women, while studies showed erectile dysfunction along with premature ejaculation is common in men.34 Tonic spasms are common in NMO/NMOSD, typically involving one side of the body or the lower extremities.35
There are also cognitive and psychiatric symptoms and concerns. The most common cognitive-impaired domains are found in learning, attention, and memory. Common psychiatric symptoms are depression and anxiety. As a result, cognitive and psychological assessments may be needed to best address these symptoms and quality of life.36-38 These symptoms all may be managed with medication and rehabilitation.
Acute Relapses
NMOSD attacks can be characterized as unpredictable, severe, and recurrent. The most prevalent ones are transverse myelitis, optic neuritis, and area postrema syndrome—inflammation or lesions of brain stem that lead to nausea, uncontrollable vomiting, hiccups, or a combination of these symptoms.39,40 These are neurologic worsening symptoms that reflect focal or multifocal CNS inflammation that develops acutely or subacutely, in the absence of fever or infection, with a duration of at least 24 hours occurring more than 30 days after the previous attack.41
Corticosteroids
In contrast to those of MS, acute attacks of NMO/NMOSD often result in permanent damage from relapses and cumulative disability.42 Therefore, aggressive, high dosages of steroids for acute management are widely accepted by most expert panel recommendations and considered the treatment mainstay. Typical dosing includes high-dose IV methylprednisolone 1,000 mg given daily for 3 to 5 days. It is known that corticosteroids induce immunosuppressive and anti-inflammatory properties by decreasing peripheral lymphocytes and reducing inflammatory cytokine, which results in reduced edema and secondary inflammation of the CNS lesion.30,42 Corticosteroid therapy can be beneficial in cases such as optic neuritis, where shortened time to treatment is associated with better visual outcomes, such as less nerve fiber loss.43 Methylprednisolone is generally well tolerated in short-term settings, but practitioners must be aware of common side effects, such as stomach ulcers, insomnia, and agitation.30
Plasma Exchange
PLEX can be considered as an adjunctive therapy for patients who present with severe symptoms, such as vision loss, those who relapsed after a prior round of PLEX, or patients who do not show adequate response to an initial burst of corticosteroids. Exchanges are carried out every other day up to a total of seven exchanges.41,44 This process is known to eliminate inflammatory cytokines and antibodies from the bloodstream that are understood to be the principal causal factors of NMO/NMOSD attacks.45 There are limited studies that argue for early initiation of PLEX as an adjunctive therapy to corticosteroids, claiming its association with better clinical outcome in patients with severe attacks defined by EDSS of >4 and/or visual acuity of <20/200.41
IV Immunoglobulins
The role of immunoglobulins (IVIG) has been studied and widely accepted in a multitude of neuroimmunologic conditions, such as myasthenia gravis and hypogammaglobulinemia. However, because of a paucity of clinical studies and evidence, the use of IVIG for acute attacks of NMOSD remains unclear at best.41 It is suggested that administration of IVIG interferes with antigen recognition, downregulates cytokine networks and adhesion molecules, and suppresses T-cell–mediated inflammation of NMO/NMOSD.46 In a study of 10 patients who did not respond to corticosteroids and PLEX, five patients showed improvement of symptoms and five did not worsen.47 Another small study found that a subset of patients who were positive for MOG-Abs refractory to steroid therapy had similar success to IVIG treatment for an acute attack.48 However, in another randomized clinical trial of a subset of patients with optic neuritis, IVIG did not show any short-term or long-term improvements.49
Nonbiologic Immunosuppressive Therapy
To date, there are not any randomized, controlled clinical trials comparing the clinical efficacy of different systemic immunosuppressants. Since available current data are obtained from a few retrospectives, observational studies, which are often confounded by hindering factors such as small sample size and retrospective case design, it is understandable that current clinical practices vary greatly between different regions and practitioner experience/preference.30
Agents such as mycophenolate, azathioprine, methotrexate, and mitoxantrone are the primary agents that have stacked the most data among other agents. Azathioprine and mycophenolate are among the most preferred choices in systemic immunosuppressants. One retrospective, nonrandomized research study examined the number of relapses among patients with NMO/NMOSD who were treated with azathioprine plus prednisone, mycophenolate only, and rituximab only for 6 months. All three of these treatment arms showed significant reduction of 72% to 88% decrease in annualized relapse rates when compared with baseline. The retrospective case design, however, is the flaw that may dwindle the strength of this clinical finding.50
However, since NMO/NMOSD is linked with very poor prognosis in which approximately 90% of patients relapse and incur a permanent physical and/or neurologic disability, there is a need for an agent that has acceptable long-term efficacy and safety profile. There are studies that suggest long-term use of nonbiologic systemic immunosuppressants may be associated with higher rates of relapse and greater risk of treatment toxicities. Monoclonal antibodies such as rituximab and other agents that earned FDA indication for chronic management of NMO/NMOSD, such as eculizumab, inebilizumab, and satralizumab, may be the solution that fits the bill.30
Biologic Immunosuppressive Therapy
The use of biologic immunosuppression to prevent relapses in NMOSD is becoming more prevalent while simultaneously becoming more costly. The biologic agents eculizumab, inebilizumab, satralizumab, rituximab, and tocilizumab are used, with studies showing them to be clinically significant.51-54 The cost of these medications varies vastly, with eculizumab incurring an initial cost of about $728,136 compared with the cost of rituximab biosimilars of around $15,000 per year.55,56 Adverse effects and drug-specific details, such as dosage and administration, may influence treatment preferences.56 (See TABLE 3.)
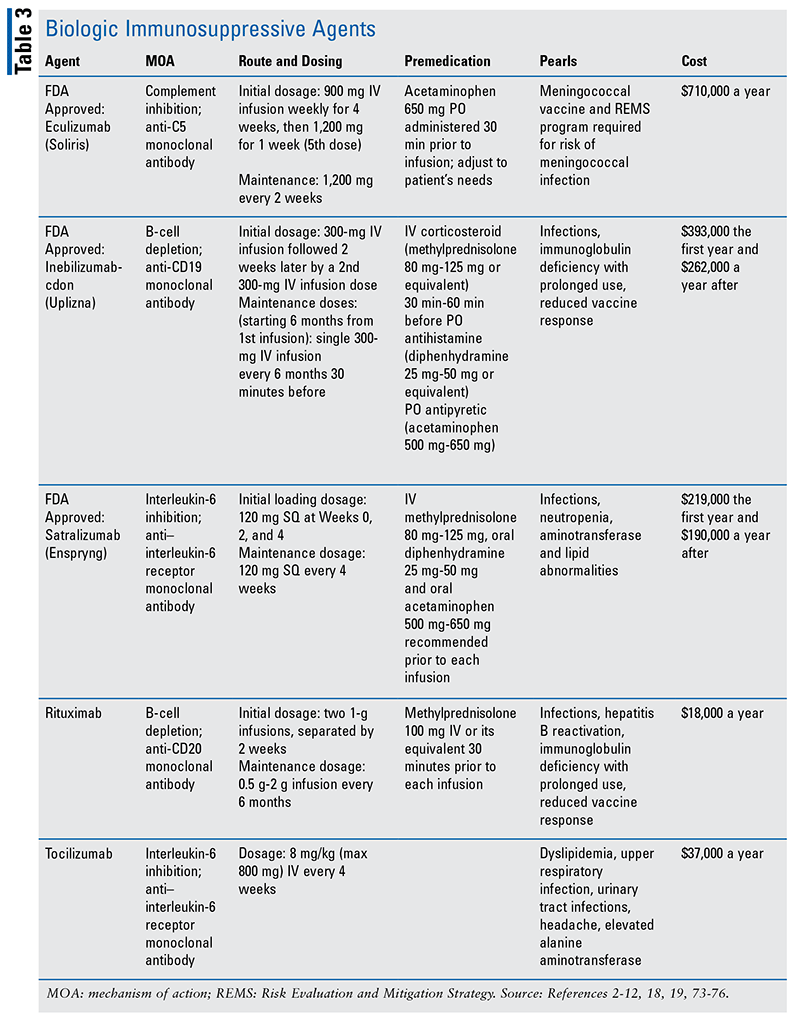
Eculizumab: Eculizumab is a complement inhibitor of protein C5, preventing the cleavage into C5a and C5b. Eculizumab was the first monoclonal antibody FDA approved for the treatment of NMO/NMOSD in adult patients who are seropositive for AQP-4 and may be considered a first-line treatment option. The Prevention of Relapses in Neuromyelitis Optica (PREVENT) trial was a pivotal study showing the effectiveness of eculizumab for the treatment of NMOSD. This trial demonstrated a decrease in relapses, the excessive daytime sleepiness scale score, and the modified Rankin scale. This trial showed 98% of patients receiving the therapy were relapse-free 144 weeks after starting treatment, compared with 45% in the placebo group.51-54,56-58 Of note, when considering eculizumab therapy, the meningococcal vaccines (serogroups ACWY and B) are required, while the Streptococcus pneumoniae and Haemophilus influenzae type b (Hib) vaccines are recommended. The streptococcus and haemophilus vaccines should be completed 2 weeks before eculizumab treatment. If these vaccinations cannot be completed 2 weeks ahead of first dose, it is recommended to start antimicrobial treatment at least 2 weeks into the treatment of eculizumab.53,57,58
Inebilizumab: Inebilizumab is a monoclonal antibody that binds to CD19 and depletes lymphocytes. Anti-CD19 monoclonal antibodies recognize and deplete a wider range of lymphocytes from the B-cell lineage compared with anti-CD20 treatments.56,59 The disadvantage of inebilizumab is that there is a delayed onset of activity against NMO/NMOSD. The N-MOmentum study showed a decrease in the relapse rate and EDSS compared with placebo in all patients, regardless of AQP4-IgG seropositivity, with 28 weeks relapse free.59 In trials, inebilizumab was not reported to treat seronegative patients. It has been observed that Ig levels may drop throughout the duration of treatment. Therefore, monitoring of IgM levels may be necessary.59 Inebilizumab should be held if there are any signs progressive multifocal leukoencephalopathy. Although not statistically significant, there have been reports of an increased risk of breast cancer, as noted in the MS ORATORIO trial.60 Patients should not take this medication if there is an active infection of hepatitis B or tuberculosis.60 Inebilizumab is about half the cost of eculizumab, and the effectiveness is somewhat comparable; however, there have not been any head-to-head trial comparisons.52,56,60
Satralizumab: Satralizumab is a humanized monoclonal antibody that targets IL-6 receptors. Satralizumab can be effective in the treatment of NMO/NMOSD due to potential spikes in IL-6 in the cerebral spinal fluid (CSF) in patients with NMOSD, therefore inhibiting IL-6, and may cause a decrease in relapse rates.61 Satralizumab was the third agent to be approved by the FDA for NMOSD.61 In the SAkuraSky trial, satralizumab was randomized to seropositive and seronegative NMOSD patients, with patients receiving therapy being relapse free at 96 weeks.61 However, this study was not powered enough to find efficacy in seronegative patients, nor was there a significant reduction in the relapse rate with the seronegative group of the study.61 Alone, nasopharyngitis, headache, upper respiratory infection, and pharyngitis are the major adverse reactions.62,63 However, in combination with other immunosuppressant medications, cellulitis becomes a more prominent adverse reaction. It is encouraged to monitor liver enzymes as well as neutrophil counts around every 4 weeks initially.62,63 Live vaccines are not recommended in patients taking satralizumab. Therefore, all live vaccines should be given at least 4 weeks before satralizumab is administered.62 Nonlive vaccines should be given 2 weeks prior to the start.62 Satrilizumab costs the least compared with the other FDA-approved drugs, and like the other FDA-approved agents, there are no head-to-head comparison studies.51,52,56
Rituximab: Rituximab, a chimeric monoclonal antibody specific for the CD20 antigen on B lymphocytes, may be used to treat NMOSD.54,60 Unlike the previous medications mentioned, rituximab is not FDA approved for NMO/NMOSD; however, many retrospective studies have been conducted, and this medication has been used for many years for the treatment of NMOSD. There have been studies showing up to 72 weeks free of relapse for patients.64 Many facilities may consider the use of rituximab as first-line therapy due to experience with the medication, cost-effectiveness, and well-established, long-term efficacy and tolerability.63-65 Meta-analysis studies have shown that rituximab tends to be safer than other immunosuppressants in NMOSD, and adverse effects were typically mild or moderate and could be well controlled.66 There have not been head-to-head studies to compare the safety and efficacy of the three FDA-approved medications (inebilizumab, satralizumab, and eculizumab) and rituximab in NMOSD.52 Vaccines should be administered at least 4 weeks prior to administration of rituximab.67 Lastly, rituximab is perhaps the most cost-effective monoclonal antibody, especially with the introduction of biosimilars.52,55
Tocilizumab: Tocilizumab is a humanized IL-6 receptor antagonist. Although tocilizumab is not FDA approved for NMO/NMOSD, studies have shown that tocilizumab reduces the level of AQP-4 Abs in the CSF.68 Tocilizumab may be used for refractory/third-line therapy.69 There have been studies showing that tocilizumab is effective against patients who have aggressive NMOSD, particularly for individuals who are refractory to rituximab (an anti-CD20 medication).70 The use of tocilizumab with rituximab does not increase immunosuppression for patients.70 Tocilizumab may cause dyslipidemia but is well tolerated.70 The most common adverse effects are upper respiratory infection, urinary tract infections, headache, hypertension, elevated alanine aminotransferase, and injection-site reactions.70 Notably, tocilizumab is not as expensive as the FDA-approved agents.71
The Pharmacist’s Role
Clinical pharmacists in a variety of practice settings, including but not limited to managed care, hospital, and ambulatory centers, are involved in the utilization and management of patients with NMO/NMOSD. Managed care pharmacists research and review NMO/NMOSD diagnostic criteria and its treatment options using any standard-of-care, clinical practice guidelines, peer-reviewed literature, FDA approvals, specialist recommendations, and any coding implications to develop clinical criteria for the prior authorization process. This helps determine the most cost-effective treatment for patients and providers along with drug billing and reimbursement. Hospital pharmacists will prepare these IV or SC NMO/NMOSD therapies for accurate dosing and administration, along with ensuring all premedications (such as acetaminophen and corticosteroids) are administered. Along with the patient’s prescriber, they also monitor disease progression, applicable laboratory values (e.g., liver function tests), contraindications, and drug-based adverse events.
Due to the complexity of this disease, pharmacists in the ambulatory setting serve as a resource for NMO/NMOSD drug therapy information and recommendations. They also provide real-time patient education on medication administration and adherence, especially for any oral and self-injectable therapies. In addition, due to the high cost and specialty categorization of these medications, these clinical pharmacists also can assist with the prior authorizations and appeals process for the treating practitioner. As in other disease-management specialties, clinical pharmacists should be key references in the interdisciplinary team of healthcare professionals.
REFERENCES
1. Paul S, Mondal GP, Bhattacharyya R, et al. Neuromyelitis optica spectrum disorders. J Neurol Sci. 2021;420:117225.
2. Huda S, Whittam D, Bhojak M, et al. Neuromyelitis optica spectrum disorders. Clin Med (Lond). 2019;19(2):169-176.
3. Jacob A, McKeon A, Nakashima I, et al. Current concept of neuromyelitis optica (NMO) and NMO spectrum disorders. J Neurol Neurosurg Psychiatry. 2013;84(8):922-930.
4. Fujihara K. Neuromyelitis optica spectrum disorders: still evolving and broadening. Curr Opin Neurol. 2019;32(3):385-394.
5. Sellner J, Boggild M, Clanet M, et al. EFNS guidelines on diagnosis and management of neuromyelitis optica. Eur J Neurol. 2010;17(8):1019-1032.
6. Oh J, Levy M. Neuromyelitis optica: an antibody-mediated disorder of the central nervous system. Neurol Res Int. 2012;2012:460825.
7. Abboud H, Salazar-Camelo A, George N, et al; on behalf of the Guthy-Jackson Foundation NMO International Clinical Consortium. Symptomatic and restorative therapies in neuromyelitis optica spectrum disorders. J Neurol. 2022;269(4):1786-1801.
8. Jarius S, Wildemann B, Paul F. Neuromyelitis optica: clinical features, immunopathogenesis and treatment. Clin Exp Immunol. 2014;176(2):149-164.
9. Kim SM, Kim SJ, Lee HJ, et al. Differential diagnosis of neuromyelitis optica spectrum disorders. Ther Adv Neurol Disord. 2017;10(7):265-289.
10. National Multiple Sclerosis Society. Neuromyelitis optica spectrum disorder (NMOSD). August 2022. https://www.nationalmssociety.org/What-is-MS/Related-Conditions/Neuromyelitis-Optica-(NMO). Accessed January 4, 2023.
11. Hor JY, Asgari N, Nakashima I, et al. Epidemiology of neuromyelitis optica spectrum disorder and its prevalence and incidence worldwide. Front Neurol. 2020;11:501.
12. Orpha.net. Neuromyelitis optica spectrum disorder. July 2021. www.orpha.net/consor/cgibin/OC_Exp.php?lng=en&Expert=71211#:~:text=Neuromyelitis%20optica%20spectrum%20disorder%20(NMOSD,of%20affected%20individuals%20are%20female. Accessed January 4, 2023.
13. Royston M, Kielhorn A, Weycker D, et al. Neuromyelitis optica spectrum disorder: clinical burden and cost of relapses and disease-related care in US clinical practice. Neurol Ther. 2021;10(2):767-783.
14. Carnero Contentti E, Correale J. Neuromyelitis optica spectrum disorders: from pathophysiology to therapeutic strategies. J Neuroinflammation. 2021;18(1):208.
15. The principles of nerve cell communication. Alcohol Health Res World. 1997;21(2):107-108.
16. Kim Y, Park J, Choi YK. The role of astrocytes in the central nervous system focused on BK channel and heme oxygenase metabolites: a review. Antioxidants (Basel). 2019;8(5):121.
17. Chiareli RA, Carvalho GA, Marques BL, et al. The role of astrocytes in the neurorepair process. Front Cell Dev Biol. 2021;9:665795.
18. Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7(1):a020412.
19. Bonomini F, Rezzani R. Aquaporin and blood brain barrier. Curr Neuropharmacol. 2010;8(2):92-96. Erratum in: Curr Neuropharmacol. 2012;10(2):179.
20. Papadopoulos MC, Verkman AS. Aquaporin 4 and neuromyelitis optica. Lancet Neurol. 2012;11(6):535-544.
21. National Cancer Institute. Lymphocyte. www.cancer.gov/publications/dictionaries/cancer-terms/def/lymphocyte. Accessed January 4, 2023.
22. LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112(5):1570-1580.
23. Pierce SK. Understanding B cell activation: from single molecule tracking, through Tolls, to stalking memory in malaria. Immunol Res. 2009;43(1-3):85-97.
24. Allen HC, Sharma P. Histology, plasma cells. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. www.ncbi.nlm.nih.gov/books/NBK556082/. Accessed January 4, 2023.
25. Asavapanumas N, Tradtrantip L, Verkman AS. Targeting the complement system in neuromyelitis optica spectrum disorder. Expert Opin Biol Ther. 2021;21(8):1073-1086.
26. Kleiter I, Gold R. Present and future therapies in neuromyelitis optica spectrum disorders. Neurotherapeutics. 2016;13(1):70-83.
27. Trebst C, Jarius S, Berthele A, et al; Neuromyelitis Optica Study Group (NEMOS). Update on the diagnosis and treatment of neuromyelitis optica: recommendations of the Neuromyelitis Optica Study Group (NEMOS). J Neurol. 2014;261(1):1-16.
28. Wingerchuk DM, Banwell B, Bennett JL, et al. International Panel for NMO Diagnosis. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177-189.
29. Collongues N, de Seze J. Current and future treatment approaches for neuromyelitis optica. Ther Adv Neurol Disord. 2011;4(2):111-121.
30. Sherman E, Han MH. Acute and chronic management of neuromyelitis optica spectrum disorder. Curr Treat Options Neurol. 2015;17(11):48.
31. Asseyer S, Cooper G, Paul F. Pain in NMOSD and MOGAD: a systematic literature review of pathophysiology, symptoms, and current treatment strategies. Front Neurol. 2020;11:778./
32. Seok JM, Choi M, Cho EB, et al. Fatigue in patients with neuromyelitis optica spectrum disorder and its impact on quality of life. PLoS One. 2017;12(5):e0177230.
33. Gupta A, Sivaram A, Krishnan R, et al. Urinary symptoms and bladder dysfunction in patients with neuromyelitis optica spectrum disorders: evaluation with urodynamics and management. J Neurosci Rural Pract. 2020;11(2):245-249.
34. Zhang Y, Zhang Q, Shi Z, et al. Sexual dysfunction in patients with neuromyelitis optica spectrum disorder. J Neuroimmunol. 2020;338:577093.
35. Abaroa L, Rodríguez-Quiroga SA, Melamud L, et al. Tonic spasms are a common clinical manifestation in patients with neuromyelitis optica. Arq Neuropsiquiatr. 2013;71(5):280-283.
36. Lopez-Soley E, Meca-Lallana JE, Llufriu S, et. al. Cognitive performance and health-related quality of life in patients with neuromyelitis optica spectrum disorder. J Pers Med. 2022;12(5):743.
37. Oertel FC, Schließeit J, Brandt AU, Paul F. Cognitive impairment in neuromyelitis optica spectrum disorders: a review of clinical and neuroradiological features. Front Neurol. 2019;10:608.
38. Shin JS, Kwon YN, Choi Y, et al. Comparison of psychiatric disturbances in patients with multiple sclerosis and neuromyelitis optica. Medicine (Baltimore). 2019;98(38):e17184.
39. Jarius S, Ruprecht K, Wildemann B, et al. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: a multicentre study of 175 patients. J Neuroinflammation. January 19, 2012. https://doi.org/10.1186/1742-2094-9-14, Accessed January 4, 2023.
40. Khalilidehkordi E, Clarke L, Arnett S, et al. Relapse patterns in NMOSD: evidence for earlier occurrence of optic neuritis and possible seasonal variation. Front Neurol. 2020;11:537.
41. Glisson C. Neuromyelitis optica spectrum disorders (NMOSD): treatment and prognosis. In: Post TW, ed. UpToDate. 2022. Accessed October 21, 2022.
42. Kessler RA, Mealy MA, Levy M. Treatment of neuromyelitis optica spectrum disorder: acute, preventive, and symptomatic. Curr Treat Options Neurol. 2016;18(1):2.
43. Nakamura M, Nakazaw, T, Doi H, et al. Early high-dose intravenous methylprednisolone is effective in preserving retinal nerve fiber layer thickness in patients with neuromyelitis optica. Graefes Arch Clin Exp Ophthalmol. 2010;248(12):1777-1785.
44. Abboud H, Petrak A, Mealy M, et al. Treatment of acute relapses in neuromyelitis optica: steroids alone versus steroids plus plasma exchange. Mult Scler. 2016;22(2):185-192.
45. Lehmann HC, Hartung H-P, Hetzel GR, et al. Plasma exchange in neuroimmunological disorders. Arch Neurol. 2006;63:1066.
46. Altunrende B, Akdal G, Bajin MS, et al. Intravenous immunoglobulin treatment for recurrent optic neuritis. Noro Psikiyatr Ars. 2019;56(1):3-6.
47. Elsone L, Panicker J, Mutch K, et al. Role of intravenous immunoglobulin in the treatment of acute relapses of neuromyelitis optica: experience in 10 patients. Mult Scler. 2014;20(4):501-504.
48. Srisupa-Olan T, Siritho S, Kittisares K, et al. Beneficial effect of plasma exchange in acute attack of neuromyelitis optica spectrum disorders. Mult Scler Relat Disord. 2018;20:115-121.
49. Noseworthy JH, O’Brien PC, Petterson TM, et al. A randomized trial of intravenous immunoglobulin in inflammatory demyelinating optic neuritis. Neurology. 2021;56(11):1514-1522.
50. Mealy MA, Wingerchuk DM, Palace J, et al. Comparison of relapse and treatment failure rates among patients with neuromyelitis optica: multicenter study of treatment efficacy. JAMA Neurol. 2014;71(3):324-330.
51. Lopez MA. Neuromyelitis optica spectrum disorder guideline recommendations. RareDiseaseAdvisor.com November 1, 2021. www.rarediseaseadvisor.com/disease-info-pages/neuromyelitis-optica-spectrum-disorder-guideline-recommendations/. Accessed January 4, 2023.
52. Cree BAC, Greenberg B, Cameron C, Weinshenker BG. Letter to the editor regarding “Network meta-analysis of Food and Drug Administration-approved treatment options for adults with aquaporin-4 immunoglobulin G-positive neuromyelitis optica spectrum disorder.” Neurol Ther. 2022;11(3):1439-1443.
53. Cleveland Clinic. Eculizumab (Soliris) for neuromyelitis optica spectrum disorder. https://my.clevelandclinic.org/departments/neurological/depts/multiple-sclerosis/ms-approaches/eculizumab#:~:text=Dosing%20regimen%20consists%20of%3A,dose%20every%202%20weeks%20afterwards. Accessed January 4, 2023.
54. Holroyd KB, Manzano GS, Levy M. Update on neuromyelitis optica spectrum disorder. Curr Opin Ophthalmol. 2020;31(6):462-468.
55. Pharmacoeconomic Report: Eculizumab (Soliris): Alexion Pharma Canada Corp. Indication: Neuromyelitis optica spectrum disorder [Internet] Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; October 2020. Appendix 1, Cost Comparison Table. www.ncbi.nlm.nih.gov/books/NBK567499/. Accessed January 4, 2023.
56. Mayo Clinic. Neurology and neurosurgery: neuromyelitis optica: new therapies offer hope. www.mayoclinic.org/medical-professionals/neurology-neurosurgery/news/neuromyelitis-optica-new-therapies-offer-hope/mac-20515747. Accessed January 4, 2023.
57. CDC. Meningococcal disease. Certain medical conditions as a risk factor. www.cdc.gov/meningococcal/about/risk-medical.html. Accessed January 4, 2023.
58. Soliris. If you are still experiencing anti-ACHR antibody-positive GMG symptoms, it may be time to consider another treatment option. https://solirisgmg.com/. Accessed January 4, 2023.
59. Diez Gaspar D. Uplizna (inebilizumab-Cdon). RareDiseaseAdvisor.com. February 17, 2022. https://www.rarediseaseadvisor.com/therapies/uplizna-inebilizumab-cdon/. Accessed January 4, 2023.
60. Brod SA. Review of approved NMO therapies based on mechanism of action, efficacy and long-term effects. Mult Scler Relat Disord. 2020;46:102538.
61. Traboulsee A, Greenberg BM, Bennett JL, et al. Safety and efficacy of satralizumab monotherapy in neuromyelitis optica spectrum disorder: a randomised, double-blind, multicentre, placebo-controlled phase 3 trial. Lancet Neurol. 2020;19(5):402-412.
62. Enspryng (satralizumab) side effects & trial safety. www.enspryng-hcp.com/side-effects/summary.html. Accessed January 4, 2023.
63. Texas Health and Human Services. Satralizumab-Mwge (Enspryng) New Drug Update. Magellan Rx Management. September 20, 2020. www.hhs.texas.gov/regulations/forms.
64. Chan KH, Lee CH. Treatment of neuromyelitis optica spectrum disorders. Int J Mol Sci. 2021;22(16):8638.
65. Gao F, Chai B, Gu C, et al. Effectiveness of rituximab in neuromyelitis optica: a meta-analysis. BMC Neurol. 2019;19(1):36.
66. Wang H, Zhou J, Li Y, et al. Adverse events of rituximab in neuromyelitis optica spectrum disorder: a systematic review and meta-analysis. Ther Adv Neurol Disord. 2021;14:17562864211056710. Erratum in: Ther Adv Neurol Disord. 2022;15:17562864221100917.
67. Kant S, Kronbichler A, Salas A, et al. Timing of COVID-19 vaccine in the setting of anti-CD20 therapy: a primer for nephrologists. Kidney Int Rep. 2021;6(5):1197-1199.
68. Du CH, Zeng P, Han JR, et al. Early initiation of tocilizumab treatment against moderate-to-severe myelitis in neuromyelitis optica spectrum disorder. Front Immunol. 2021;12:660230.
69. Zhang C, Zhang M, Qiu W, et al; TANGO Study Investigators. Safety and efficacy of tocilizumab versus azathioprine in highly relapsing neuromyelitis optica spectrum
disorder (TANGO): an open-label, multicentre, randomised, phase 2 trial. Lancet Neurol. 2020;19(5):391-401.70. Sawaya R, Saab G, Moussa H. Should tocilizumab be the first line treatment for neuromyelitis optica together with rituximab? Neurologia (Engl Ed). 2021;36(8):642-643.
71. Jogimahanti AV, Kini AT, Irwin LE, Lee AG. The cost-effectiveness of tocilizumab (Actemra) therapy in Giant Cell Arteritis. J Neuroophthalmol. 2021;41(3):342-350.
72. Eculizumab. IBM Micromedex Solutions. Ann Arbor, MI: Truven Health Analytics, Inc. www.micromedexsolutions.com/micromedex2/librarian/CS/B1A07F/ND_PR/evidencexpert/ND_P/evidencexpert/DUPLICATIONSHIELDSYNC/CB748C/ND_PG/evidencexpert/ND_B/evidencexpert/ND_AppProduct/evidencexpert/ND_T/evidencexpert/PFActionId/evidencexpert.DoIntegratedSearch?SearchTerm=Eculizumab%20&UserSearchTerm=Eculizumab%20&SearchFilter=filterNone&navitem=searchALL#. Accessed October 24, 2022.
73. Inebilizumab. IBM Micromedex Solutions. Ann Arbor, MI: Truven Health Analytics, Inc. www.micromedexsolutions.com/micromedex2/librarian/CS/5FAAE2/ND_PR/evidencexpert/ND_P/evidencexpert/DUPLICATIONSHIELDSYNC/A2B53D/ND_PG/evidencexpert/ND_B/evidencexpert/ND_AppProduct/evidencexpert/ND_T/evidencexpert/PFActionId/evidencexpert.DoIntegratedSearch?SearchTerm=Inebilizumab%20&UserSearchTerm=Inebilizumab%20&SearchFilter=filterNone&navitem=searchGlobal#. Accessed October 24, 2022.
74. Satralizumab. IBM Micromedex Solutions. Ann Arbor, MI: Truven Health Analytics, Inc. www.micromedexsolutions.com/micromedex2/librarian/CS/E9C1AE/ND_PR/evidencexpert/ND_P/evidencexpert/DUPLICATIONSHIELDSYNC/BB6C72/ND_PG/evidencexpert/ND_B/evidencexpert/ND_AppProduct/evidencexpert/ND_T/evidencexpert/PFActionId/evidencexpert.DoIntegratedSearch?SearchTerm=Satralizumab&UserSearchTerm=Satralizumab&SearchFilter=filterNone&navitem=searchALL#. Accessed October 24, 2022.
75. Rituximab. IBM Micromedex Solutions. Ann Arbor, MI: Truven Health Analytics, Inc. www.micromedexsolutions.com/micromedex2/librarian/CS/FCA3AE/ND_PR/evidencexpert/ND_P/evidencexpert/DUPLICATIONSHIELDSYNC/350448/ND_PG/evidencexpert/ND_B/evidencexpert/ND_AppProduct/evidencexpert/ND_T/evidencexpert/PFActionId/evidencexpert.DoIntegratedSearch?SearchTerm=Rituximab&fromInterSaltBase=true&UserMdxSearchTerm=%24userMdxSearchTerm&false=null&=null#. Accessed October 24, 2022.
76. Tocilizumab. IBM Micromedex Solutions. Ann Arbor, MI: Truven Health Analytics, Inc. www.micromedexsolutions.com/micromedex2/librarian/CS/FB4396/ND_PR/evidencexpert/ND_P/evidencexpert/DUPLICATIONSHIELDSYNC/816841/ND_PG/evidencexpert/ND_B/evidencexpert/ND_AppProduct/evidencexpert/ND_T/evidencexpert/PFActionId/evidencexpert.DoIntegratedSearch?SearchTerm=Tocilizumab&UserSearchTerm=Tocilizumab&SearchFilter=filterNone&navitem=searchGlobal#. Accessed October 24, 2022.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






