US Pharm. 2012;37(3):50-51.
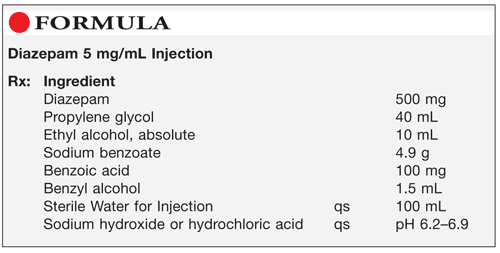
Method of Preparation: Note—This formulation should be prepared according to strict aseptic compounding technique in a laminar airflow hood in a cleanroom or via isolation barrier technology by a compounding pharmacist validated in aseptic compounding. This is a high-risk preparation.
Calculate the required quantity of each ingredient for the total amount to be prepared. Accurately weigh or measure each ingredient. Mix the propylene glycol, alcohol, and benzyl alcohol. Dissolve the diazepam and benzoic acid in the solution. Slowly add Sterile Water for Injection to a volume of 75 to 80 mL. Add the sodium benzoate and mix until dissolved; then add the sodium hydroxide solution or hydrochloric acid to adjust the pH to the range of 6.2 to 6.9. Add sufficient Sterile Water for Injection to volume and mix well. Filter through an appropriate sterile 0.2-µm filter into sterile vials. Package and label.
Use: Diazepam is a benzodiazepine that has anticonvulsant, anxiolytic, sedative, muscle relaxant, and amnesic properties.2
Packaging: Package in sterile glass vials.
Labeling: For professional use.
Stability: If no sterility-testing program is in place, a beyond-use date of up to 24 hours may be used if this product is stored at controlled room temperature, 3 days if stored in a refrigerator, or 45 days in solid frozen state at −25°C to −10°C or colder. If a sterility-testing program is in place, a beyond-use date of up to 6 months may be used for this preparation.1
Quality Control: Quality-control assessment can include weight and/or volume, physical observation, pH, specific gravity, osmolality, assay, color, clarity, particulate matter, sterility, and pyrogenicity.3,4
Discussion: Because of the large number of drug shortages, compounding pharmacists are filling the gap to meet patients’ needs. Many hospitals are outsourcing the compounding of needed medications to USP <797>–compliant pharmacies.
Diazepam (Valium, C16H13ClN2O, MW 284.75) occurs as an off-white to yellow, practically odorless, crystalline powder. It is practically insoluble in water (1 g in 333 mL water), but soluble in alcohol (1 g in 16 mL). Diazepam melts between 131°C and 135°C.1,2
Diazepam injection USP is a sterile solution of diazepam in a suitable medium. It has a pH between 6.2 and 6.9, contains not more than 11.6 USP Endotoxin Units per mg of diazepam, and has an osmolality of about 7,775 mOsm/kg.1 Because of the cosolvent system used to solubilize the diazepam, care should be taken when diluting this injection.
Propylene glycol (C3H8O2) occurs as a clear, colorless, viscous, practically odorless liquid with a sweet taste, somewhat resembling glycerin. It has a specific gravity of 1.038 g/mL, and it is miscible with 95% ethanol, glycerin, and water.5
Alcohol (ethyl alcohol, ethanol, grain alcohol, C2H5OH, MW 46.07) is a clear, colorless, mobile, volatile liquid with a slight, characteristic odor and a burning taste. Alcohol USP refers to 95% ethanol, and dehydrated alcohol refers to 99.5% alcohol. Its specific gravity is between 0.812 and 0.816, and its boiling point is 78.15°C. Alcohol is miscible with glycerin and water.6
Sodium benzoate (C7H5NaO2, MW 144.11) occurs as a white granular or crystalline, slightly hygroscopic powder. It is generally odorless and has an unpleasant sweet and salty taste. Sodium benzoate is soluble 1 g in 1.8 mL of water and 75 mL of 95% ethanol. It is used as an antimicrobial preservative.7
Benzoic acid (C7H6O2, MW 122.12) occurs as white crystals, scales, or needles with a slight odor. It is somewhat volatile at warmer temperatures, slightly soluble in water, and freely soluble in alcohol.1
Benzyl alcohol (C7H8O, MW 108.14) is an antimicrobial preservative, disinfectant, and solvent that occurs as a clear, colorless, oily liquid that has a faint, aromatic odor and a sharp, burning taste. Benzyl alcohol has a specific gravity of about 1.045. It is soluble 1 g in 25 mL of water at 25°C and is miscible with ethanol.8
REFERENCES
1. U.S. Pharmacopeia 34/NF 29. Rockville, MD: U.S. Pharmacopeial Convention, Inc; 2011:330-336,336-373,1009,2533-2536.
2. McEvoy GK, ed. ASHP Drug Information 2011. Bethesda, MD: American Society of Health-Systems Pharmacists; 2011:2620-2623.
3. Allen LV Jr. Standard operating procedure for particulate testing for sterile products. IJPC. 1998;2:78.
4. Allen LV Jr. Standard operating procedure: quality assessment for injectable solutions. IJPC. 1999;3:406-407.
5. Weller PJ. Propylene glycol. In: Rowe RC, Sheskey PJ, Quinn ME, eds. Handbook of Pharmaceutical Excipients. 6th ed. London, England: Pharmaceutical Press; 2009:592-594.
6. Quinn ME. Alcohol. In: Rowe RC, Sheskey PJ, Quinn ME, eds. Handbook of Pharmaceutical Excipients. 6th ed. London, England: Pharmaceutical Press; 2009:17-19.
7. Sakurai T. Sodium benzoate. In: Rowe RC, Sheskey PJ, Quinn ME, eds. Handbook of Pharmaceutical Excipients. 6th ed. London, England: Pharmaceutical Press; 2009:627-629.
8. Storey RA. Benzyl alcohol. In: Rowe RC, Sheskey PJ, Quinn ME, eds. Handbook of Pharmaceutical Excipients. 6th ed. London, England: Pharmaceutical Press; 2009:64-66.
To comment on this article, contact rdavidson@uspharmacist.com.





