US Pharm. 2021;46(3):60-61.
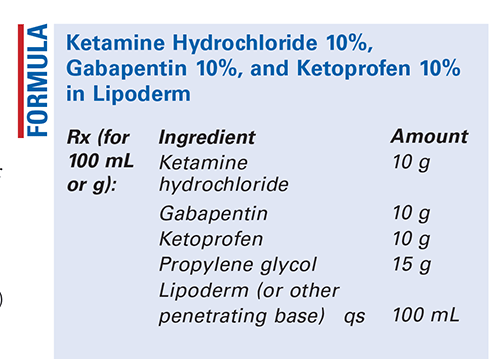
Method of Preparation: Calculate the required quantity of each ingredient for the total amount to be prepared. Accurately weigh or measure each ingredient. Blend the powders together thoroughly. Add the propylene glycol and mix well. Geometrically, incorporate the Lipoderm or other penetrating base of choice with thorough mixing after each addition. Package and label.
Use: This preparation has been used in the treatment of complex regional pain syndrome (CRPS) and other pain disorders.1
Packaging: Package in tight, light-resistant containers.
Labeling: Keep out of reach of children. Discard after ____ [time period].
Stability: A beyond-use date of 30 days may be used if water is present in the penetrating base, or up to 6 months if the base is anhydrous.2
Quality Control: Quality-control assessment can include theoretical weight compared with actual weight, specific gravity, active drug assay, color, texture–surface, texture–spatula spread, appearance, feel, rheologic properties, and physical observations.3-5
Discussion: CRPS is a progressive, multifactorial condition that usually develops in a limb following trauma or surgery. Treatments for this condition are limited, so the use of topical ketamine and other agents is promising, especially given the favorable safety profile of topical agents.1
Ketamine hydrochloride (C13H16ClNO.HCl, MW 274.2) occurs as a white, crystalline powder with a slight, characteristic odor. Approximately 1.15 mg is equivalent to 1 mg of ketamine base. Ketamine hydrochloride is soluble 1 g in 4 mL of water, in 14 mL of alcohol, and in 60 mL of absolute alcohol. It should be stored at controlled room temperature and protected from light. Ketamine hydrochloride is a general anesthetic, and it has been used topically as an analgesic in concentrations from 0.5% to 20% and in combination with other analgesics.1,6
Gabapentin (Neurontin, cyclohexaneacetic acid, C9H17NO2, MW 171.24) occurs as a white to off-white, crystalline solid that is freely soluble in water and in alkaline and acidic solutions. A 2% aqueous solution has a pH of 6.5 to 8.0. Gabapentin is an anticonvulsant that is structurally related to gamma-aminobutyric acid. It is used in combination with other anticonvulsant agents in the management of seizure disorders, neuropathic pain, vasomotor symptoms, and other disorders.1,7
Ketoprofen (C16H14O3, MW 254.28) occurs as a white or almost white, odorless or almost odorless, crystalline powder. It is practically insoluble in water but is freely soluble in alcohol and ether. It has a melting range of 92.0°C to 97.0°C. Ketoprofen has analgesic, anti-inflammatory, and antipyretic properties, and it is an inhibitor of cyclooxygenase. Ketoprofen should be preserved in tight containers.1
Propylene glycol (C3H8O2) occurs as a clear, colorless, viscous, practically odorless liquid with a sweet taste that somewhat resembles glycerin. It has a specific gravity of 1.038 g/mL. Propylene glycol is miscible with acetone, chloroform, 95% ethanol, glycerin, and water.8
Lipoderm is an elegant alternative to traditional pluronic lecithin organogels (PLOs) that has a smooth and creamy feel, in contrast to the tacky feel of PLOs. It contains a proprietary liposomal component that may increase the permeation of a variety of actives.9
REFERENCES
1. Durham MJ, Mekhjian HS, Goad JA, et al. Topical ketamine in the treatment of complex regional pain syndrome. IJPC. 2018;22:172-175.
2. U.S. Pharmacopeia/National Formulary [current revision]. Rockville, MD: U.S. Pharmacopeial Convention, Inc; February 2021.
3. Allen LV Jr. Standard operating procedure for performing physical quality assessment of ointments/creams/gels. IJPC. 1998;2:308-309.
4. Allen LV Jr. Summary of quality-control testing for sterile and nonsterile compounded preparations, part 1: physical and chemical testing. IJPC. 2019;23:211-216.
5. Allen LV Jr. Summary of quality-control testing for sterile and nonsterile compounded preparations, part 2: microbiological testing. IJPC. 2019;23:299-303.
6. Sweetman SC, ed. Martindale: The Complete Drug Reference. 33rd ed. London, England: Pharmaceutical Press; 2002:1262-1263.
7. Sweetman SC, ed. Martindale: The Complete Drug Reference. 36th ed. London, England: Pharmaceutical Press; 2009:482-484.
8. Driver S. Propylene glycol. In: Sheskey PJ, Hancock BC, Moss GP, Goldfarb DJ, eds. Handbook of Pharmaceutical Excipients. 9th ed. London, England: Pharmaceutical Press; 2021:867-870.
9. Professional Compounding Centers of America. Lipoderm. www.pccarx.com. Accessed February 1, 2021.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





