US Pharm. 2010;35(5):44-50.
On behalf of patient safety considerations, providers must receive the support for safely switching patients to and from oral fentanyl products. This could be accomplished with the upcoming implementation of the Risk Evaluation Mitigation Strategy (REMS) for opioids.
Risk Evaluation Mitigation Strategy
REMS, under the authority of the FDA, is designed to manage known or potentially serious drug effects.1 To ensure safe use, the REMS program may include but is not limited to training or certification for prescribing or dispensing, dispensing under a particular circumstance, requiring patient registries, or instituting monitoring programs.2 TABLE 1 provides directions to make a formal comment to the FDA about this topic.3 The FDA and all health care providers should propose that the manufacturers of the oral fentanyl products be required to provide due diligence through clinical studies to evaluate and estimate oral morphine equivalent doses and provide guidelines and/or training for safely switching to and, more importantly, switching from oral fentanyl products.
The use of the oral fentanyl products off-label for noncancer breakthrough pain has resulted in a largely untrained group of health care professionals prescribing and dispensing these difficult-to-dose opioids. The focus of this paper will be on switching from the ultra-short-acting oral fentanyl products (Actiq and its generic lozenge and the Fentora buccal tablet), since this is where patient safety and data for safe conversions (switching) are lacking or are confusing.

Difficulties Switching From Oral Fentanyl Products
One of the main difficulties with safely switching from the ultra-short-acting oral fentanyl products has to do with a lack of conversion data for opioids and particularly for oral fentanyl. Much of the conversion data available for switching between opioids was obtained from single-dose crossover studies in opioid-naïve patients with acute pain.4 This makes it important to recognize the limitations of the data if being used for chronic breakthrough pain. Manufacturers of the oral fentanyl products have provided data to switch to the oral fentanyl products but have not provided safe conversion data or training to switch from the oral fentanyl product to a nonfentanyl opioid.5-7 The practitioner is left in a predicament to “guestimate” on the best strategy for switching from these agents. The results of an inaccurate switch can be an overdose or an underdose, either of which can result in consequences to the practitioner and the patient. It is necessary that the experts on these products, the manufacturers, provide data and training for safely switching from these agents.
Another difficulty with switching from these agents may include patient preference. The high lipophilicity of fentanyl and its ability to quickly cross the blood–brain barrier enhances the potential for abuse or misuse. According to the U.S. Department of Justice, Drug Enforcement Administration Office of Diversion Control, fentanyl is more potent than heroin.8 When a patient has a high preference for an opioid and the provider does not have adequate tools or knowledge for safely switching, it may be challenging to move the patient away from these opioids. As part of the REMS program, it may be advisable to train providers on issues related to dependence, addiction, and abuse.
Opioid Switching
Opioid switching may be desired under certain circumstances such as management of medication-related adverse events and optimization and management of pain control in patients with complex or challenging pain conditions. Other reasons for switching include possible abuse or diversion of a particular opioid, change in patient status, pharmacy availability, and excessive cost when used for long-term, chronic pain.4,9 It is important to consider that studies have shown that many patients must switch opioid medications at least once, sometimes as many as three to four times, before achieving effective analgesia.9 For these reasons, appropriate guidelines and training for switching from the ultra-short-acting oral fentanyl products to a nonfentanyl opioid are needed.
The strategy for switching from Actiq and Fentora to a nonfentanyl opioid in this article is the sole opinion of the author. Neither Actiq, nor Fentora, nor Onsolis (fentanyl buccal soluble film) are equianalgesic to each other.5-7 It is recommended that all primary literature and data obtained from this article be evaluated and critiqued for accuracy and increased knowledge by anyone wishing to follow this strategy for switching. Equianalgesic doses are approximations. Prescribers are advised to closely monitor the patient undergoing an opioid switch to make needed adjustments. In TABLE 2, the conversion ratio used when converting from an oral morphine equivalent dose to oral hydromorphone was 7.5:1.10-14 However, other references refer to a conversion ratio of 5:1 when converting in this direction.4 (An example for switching from Onsolis is not included in this article.)

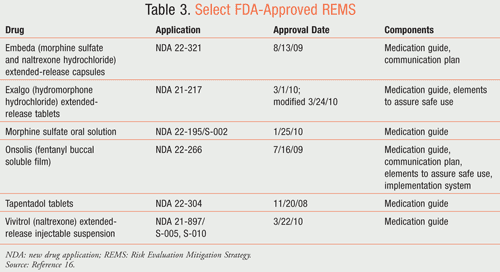
Although there is a lack of data to safely switch from Onsolis to a nonfentanyl opioid, the approved REMS program for Onsolis should minimize dosage-converting errors. According to the approved REMS, health care providers who prescribe Onsolis will be specifically certified and enrolled in the education program known as the FOCUS Program for Onsolis.15 Specialty pharmacies will also be certified and enrolled. The program does not allow for community or hospital participation. Finally, patients will be counseled and enrolled in the program as well. TABLE 3 lists medications with approved REMS (this list is not all-inclusive).16
Conclusion
The REMS program for opioids may result in an increased safety net by providing training and guidance to prescribers, pharmacists, and patients. The difficulty with switching from the oral fentanyl products to a nonfentanyl opioid is due to a lack of clinical data or guidelines for converting these agents. The increased off-label use of Actiq and Fentora for chronic noncancer pain has resulted in a larger group of untrained health care providers prescribing and dispensing these medications. Many health care providers lack the knowledge and practical experience for switching from the oral fentanyl products to a nonfentanyl opioid, which could result in an underdose, overdose, or death.
It has been proposed that the manufacturers of the oral fentanyl products develop a REMS program that provides adequate conversion data and training for switching from these drugs to a nonfentanyl opioid. All providers who dispense or prescribe oral fentanyl products are encouraged to submit their comments at www.regulations.gov. (See TABLE 1 for submission directions.3) The comment period closes on October 19, 2010.
REFERENCES
1. Shane R. Risk evaluation and mitigation strategies: impact on patients, health care providers, and health systems. Am J Health Syst Pharm. 2009;66(suppl 7):S6-S12.
2. Meyer BM. The Food and Drug Administration Amendments Act of 2007: drug safety and health-system pharmacy implications. Am J Health Syst Pharm. 2009;66 (suppl 7):S3-S5.
3. Risk evaluation and mitigation strategies for certain opioid drugs; notice of public meeting; reopening of comment period. Document ID FDA-2009-N-0143-1061. www.regulations.gov/search/
4. McPherson ML. Demystifying Opioid Conversion Calculations: A Guide for Effective Dosing.
5. Actiq (fentanyl citrate) package insert. Salt Lake City, UT: Cephalon, Inc; September 2009. www.actiq.com/pdf/actiq_ Bethesda, MD: American Society of Health-System Pharmacists; 2010:2-7.
6. Fentora (fentanyl buccal tablet) package insert. Salt Lake City, UT: Cephalon, Inc; December 2009. www.fentora.com/pdfs/pdf100_
7. Onsolis (fentanyl buccal soluble film) package insert. Somerset, NJ: Meda Pharmaceuticals Inc; July 2009. www.onsolis.com/pdf/onsolis_
8. Drugs and chemicals of concern. Fentanyl. U.S. Department of Justice, Drug Enforcement Administration Office of Diversion Control. October 2009. www.deadiversion.usdoj.gov/
9. Sinatra R. Opioid analgesics in primary care: challenges and new advances in the management of noncancer pain. J Am Board Fam Med. 2006;19:165-177.
10. Baumann TL. Chapter 58. Pain management. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 6th ed. New York, NY: McGraw-Hill; 2005:1096.
11. Cephalon, Inc. (800) 896-5855. Medical information, written communication. Analgesic potency of ACTIQ (fentanyl citrate) oral transmucosal lozenge CII relative to that of other opioid medications. February 2009.
12. VA/DoD Clinical Practice Guidelines for the Management of Opioid Therapy for Chronic Pain. Version 1.0. Department of Veterans Affairs, Department of Defense. March 2003. http://pain-topics.org/pdf/VA_
13. Darwish M, Kirby M, Robertson P, et al. Absolute and relative bioavailability of fentanyl buccal tablet and oral transmucosal fentanyl citrate. J Clin Pharmacol. 2007;47:343-350.
14. Opioid (narcotic) analgesic converter. GlobalRPh. www.globalrph.com/narcotic.cgi
15. The FOCUS Program for Onsolis. Meda Pharmaceuticals. August 2009. www.onsolisfocus.com. Accessed April 13, 2010.
16. Approved risk evaluation and mitigation strategies (REMS). Postmarket drug safety information for patients and providers. FDA. April 6, 2010. www.fda.gov/Drugs/DrugSafety/
To comment on this article, contact rdavidson@uspharmacist.com.





