US Pharm. 2018;43(12):HS7-HS11.
ABSTRACT: Intraabdominal infections is a broad term describing infections contained within the peritoneal cavity. Intraabdominal infections are classified as complicated or uncomplicated, and although their treatment is well established, clinical questions surround the optimal duration of antimicrobial therapy. Recently published evidence-based treatment guidelines recommend a shortened duration of antimicrobial treatment, which is inconsistent with traditional practice. Shortened duration of antimicrobial treatment in intraabdominal infections does not have a negative impact on patient outcomes. Pharmacists can play a vital role in the antimicrobial decision-making process by educating the healthcare team and advising them on the appropriate treatment to improve patient care.
The treatment of intraabdominal infections (IAI) is well established and based on current guidelines published by the Infectious Diseases Society of America (IDSA), the Surgical Infection Society (SIS), the 2018 Tokyo Guidelines (TG18), and the World Society of Emergency Surgery (WSES).1-4 Common antimicrobials used in the treatment of IAI include cephalosporins, fluoroquinolones, carbapenems, aminoglycosides, and penicillins. The guidelines are consistent in the recommended treatments; however, clinical questions surround the optimal duration of antimicrobial therapy.
CLASSIFICATION
IAI is a broad term describing infections that are contained within the peritoneal cavity. IAI are classified as complicated or uncomplicated. Uncomplicated IAI (uIAI), which are confined to various structures, include appendicitis, cholecystitis, and cholangitis. Complicated IAI (cIAI), such as peritonitis and abscesses, extend beyond the single organ into the peritoneal space.5,6
RECOMMENDATIONS FOR DURATION OF ANTIMICROBIAL THERAPY
See TABLE 1 for a summary of current recommendations for duration of antimicrobial therapy.1-4
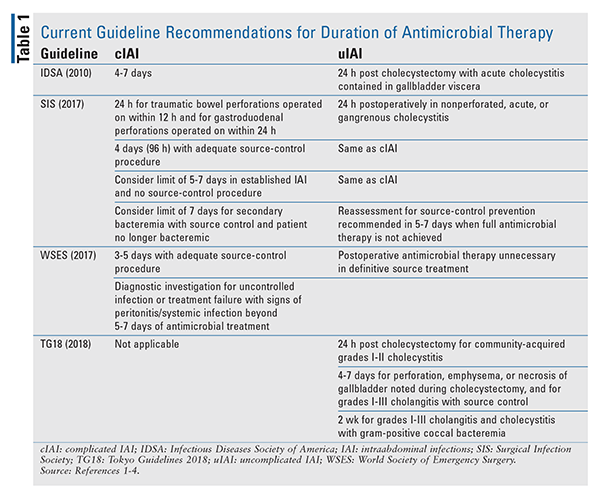
Uncomplicated IAI
The 2010 IDSA guidelines state that antibiotic therapy should be initiated in patients with suspected acute cholangitis or cholecystitis. Although the guidelines recommend discontinuing antibiotic therapy within 24 hours after cholecystectomy in patients with acute cholecystitis if the infection is contained within the viscera of the gallbladder, they fail to recommend a duration of antibiotic therapy in patients with acute cholangitis.1 In comparison, the 2017 SIS guidelines recommend a 24-hour postoperative limit of antibiotic therapy in patients with acute or gangrenous cholecystitis in the absence of perforation (grade 1A).2
The SIS guidelines provide additional guidance for patients with uIAI. In patients in whom an adequate source-control procedure was performed, no more than 4 full days (96 hours) of antimicrobial therapy are recommended (grade 1A).2 In patients who did not have an adequate source-control procedure, the recommendation is no more than 5 to 7 days of antibiotic therapy. Additionally, treatment duration should be based on assessment of such parameters as fever, leukocytosis, and gastrointestinal function. Reassessment for source-control prevention is recommended in patients who do not respond fully to antibiotic therapy within 5 to 7 days (grade 2C).2
The 2017 WSES guidelines offer little information on antibiotic therapy duration for the treatment of uIAI other than stating that postoperative antibiotic therapy is unnecessary in patients with uIAI in whom the source of infection has been definitively treated (grade 1A).4
Conversely, the TG18 guidelines offer specific therapy durations for acute cholangitis and cholecystitis based on the biliary infection’s grade and classification.3 Antibiotic therapy for community-acquired grades I and II cholecystitis should be discontinued within 24 hours of cholecystectomy, and extended therapy (4-7 days) may be warranted for specific conditions, including perforation, emphysema, or gallbladder necrosis noted during cholecystectomy. For grades I, II, and III cholangitis and grade III cholecystitis, treatment should be administered for 4 to 7 days after adequate source control, and a minimum of 2 weeks is recommended for gram-positive coccal bacteremia. A minimum duration of 2 weeks is recommended for healthcare-associated cholangitis and cholecystitis (grades I-III) in patients with gram-positive coccal bacteremia.3
Complicated IAI
Current treatment guidelines for cIAI include assessing patient risk and making a diagnosis, completing a source-control procedure, and instituting antimicrobial therapy. Although the guidelines caution that the duration of antimicrobial therapy in IAI should be patient-specific by clinical condition, distinct recommendations are given with regard to duration of therapy for appropriate antimicrobial agents.2,4 The 2010 IDSA guidelines recommended 4 to 7 days of antimicrobial therapy, but noted data suggested that the average length of therapy remain at 10 to 14 days.1 Current guidelines include recommendations for a shorter duration of therapy for cIAI.
SIS recommendations are as follows: 1) Limit antimicrobial therapy to 24 hours in patients with traumatic bowel perforations operated on within 12 hours (grade 1A) and in those with gastroduodenal perforations operated on within 24 hours (grade 1C). 2) Limit antimicrobial therapy to 4 days (96 h) in patients who had an adequate source-control procedure (grade 1A). 3) Consider limiting antimicrobial therapy to 5 to 7 days in patients with established IAI in whom a definitive source-control procedure is not performed; patients who do not fully respond to antimicrobial therapy within 5 to 7 days should be reassessed for a potential source-control intervention (grade 2C). 4) Consider limiting antimicrobial therapy to 7 days in patients who have secondary bacteremia due to IAI, have undergone adequate source control, and are no longer bacteremic (grade 2B).2
Likewise, in patients with cIAI undergoing an adequate source-control procedure (grade 1A), the WSES recommends a short course of antibiotic therapy (3-5 days), along with a diagnostic investigation to determine whether there is an ongoing uncontrolled source of infection or antimicrobial-therapy failure in patients with ongoing signs of peritonitis or systemic infection beyond 5 to 7 days of antibiotic treatment (grade 1C).4
EVIDENCE FOR SHORTENED DURATION OF THERAPY
Uncomplicated IAI
Cholecystitis: Cholecystectomy, a procedure that removes the source of infection, is often performed in patients with acute cholecystitis to prevent the likelihood of recurrence.6 In an observational study, 287 patients with grade II acute cholecystitis were grouped according to antibiotic therapy duration (0-4 days, 5-7 days, or >7 days) and were monitored for a minimum of 30 days post cholecystectomy to ascertain whether any of the therapy-duration groups had a greater frequency of surgical-site infection (SSI).7 No significant differences in frequency of SSI were noted between groups, and the study concluded that extending antibiotic therapy beyond 4 days demonstrated no benefit for reducing SSI incidence.7 In a retrospective cohort analysis, short (7 days or less) versus long (more than 7 days) courses of antibiotic therapy were compared in 81 patients with acute cholecystitis following percutaneous cholecystectomy.8 No significant differences between groups were found, and prolonged use of antibiotics conferred no advantage.8
Cholangitis: Biliary drainage plus antibiotic therapy remains the standard of care for patients with acute cholangitis.3 A 2017 retrospective cohort study assessed the effectiveness of short-course antibiotic therapy (SCT)—defined as 7 days or less—in patients with acute bacteremic cholangitis following successful biliary drainage.9 There were no significant differences in patient outcomes (30-day mortality, recurrence, complications, or worsening of symptoms), which suggests that antimicrobial SCT does not lead to worse clinical outcomes.9 A similar study sought to determine the optimal treatment duration—less than 14 days versus 14 or more days—in patients with acute cholangitis.10 There was no significant difference in 30-day mortality between the groups; however, treatment duration and hospital length of stay were shorter in the shorter-duration group (both, P <.001).10
Complicated IAI
Several clinical trials have been performed in patients with cIAI to determine whether a shorter duration of antimicrobial therapy affects outcomes.11-14 The Study to Optimize Peritoneal Infection Therapy (STOP-IT) compared a fixed antimicrobial-therapy duration of approximately 4 days with the traditional practice of antibiotic therapy through 2 days following resolution of symptoms, with a maximum therapy of 10 days.11 This open-label, multicenter, randomized trial included 518 patients with cIAI. No significant between-group differences were found with regard to the composite primary endpoint of SSI, recurrent abdominal infection, and death, and there was no difference between groups in time to the composite primary outcome.11
Based on the results of that study, two post hoc analyses were performed that used the STOP-IT patient database. One analysis was conducted to identify patients at high risk for treatment failure and to discover whether they would benefit from a longer duration of antimicrobial therapy.12 This study found no significant difference in treatment failure between the shorter-duration and longer-duration antimicrobial therapy groups.12 In the other analysis, patients with specific risk factors for complications were evaluated to determine whether outcomes were similar between the shorter-duration and longer-duration therapy groups.13 Patients who had risk factors for developing complications (i.e., obesity, diabetes) demonstrated no significant differences in outcomes.
Another study evaluated the efficacy and safety of shortened-duration antimicrobial therapy in 249 critically ill patients with postoperative IAI and adequate source control.14 All patients received therapy for 8 days, after which they were randomized to either discontinuation of therapy or continuation of therapy to a total of 15 days. The primary endpoint was the number of antibiotic-free days between day 8 and day 28. No clinical benefit was found for the additional 7 days of therapy. Additionally, no statistically significant difference in 45-day mortality rate was observed between the two treatment groups.14
THE PHARMACIST’S ROLE
By virtue of their profession, pharmacists are educators, regardless of whether the audience comprises patients or providers. The role of the pharmacist in the treatment of IAI relies heavily on educating healthcare providers about antimicrobial stewardship. The CDC estimates that more than 2 million people are infected with antibiotic-resistant organisms, resulting in 23,000 deaths per year.15 Optimal use of antimicrobial therapy is necessary to improve patient outcomes and minimize the potential for further antibiotic resistance.16,17 The duration of antimicrobial therapy following source control for cIAI presents an opportunity for antimicrobial stewardship.11 Current guidelines support stewardship measures for both uIAI and cIAI through recommendations optimizing the use and duration of antimicrobial therapy.2,4 Pharmacists can influence the antimicrobial decision-making process with respect to appropriateness and duration of therapy for IAI by providing evidence-based information to foster provider knowledge.
CONCLUSION
It is difficult to determine accurate epidemiologic data for IAI because IAI encompass a broad range of infections of the peritoneal cavity. Current guidelines recommend a shorter duration of therapy for IAI, specifically when adequate source control has been achieved. Evidence-based recommendations apply to general IAI patients, critically ill patients, and patients at higher risk for developing infectious complications and treatment failure. Pharmacists are in a unique position because they not only can educate other healthcare providers on the proper and judicious use of antibiotics, but can also advise at the point of care. Therefore, appropriate evidence-based treatment should be instituted in patients with IAI in order to achieve optimal outcomes.
REFERENCES
1. Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:133-164.
2. Mazuski JE, Tessier JM, May AK, et al. The Surgical Infection Society revised guidelines on the management of intra-abdominal infection. Surg Infect (Larchmt). 2017;18:1-76.
3. Gomi H, Solomkin JS, Schlossberg D, et al. Tokyo Guidelines 2018: antimicrobial therapy for acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci. 2018;25:3-16.
4. Sartelli M, Chichom-Mefire A, Labricciosa FM, et al. The management of intra-abdominal infections from a global perspective: 2017 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2017;12:29.
5. Olsen KM, Gross AE, DiPiro JT. Intraabdominal infections. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 9th ed. New York, NY: McGraw-Hill; 2014:1821-1834.
6. Levison ME, Bush LM. Peritonitis and intraperitoneal abscesses. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier; 2015:935-959.
7. Rodríguez-Sanjuán JC, Casella G, Antolín F, et al. How long is antibiotic therapy necessary after urgent cholecystectomy for acute cholecystitis? J Gastrointest Surg. 2013;17:1947-1952.
8. Loftus TJ, Brakenridge SC, Dessaigne CG, et al. Antibiotics may be safely discontinued within one week of percutaneous cholecystostomy. World J Surg. 2017;41:1239-1245.
9. Doi A, Morimoto T, Iwata K. Shorter duration of antibiotic treatment for acute bacteraemic cholangitis with successful biliary drainage: a retrospective cohort study. Clin Microbiol Infect. 2018;24:1184-1189.
10. Uno S, Hase R, Kobayashi M, et al. Short-course antimicrobial treatment for acute cholangitis with Gram-negative bacillary bacteremia. Int J Infect Dis. 2017;55:81-85.
11. Sawyer RG, Claridge JA, Nathens AB, et al. Trial of short-course antimicrobial therapy for intraabdominal infection. N Engl J Med. 2015;372:1996-2005.
12. Hassinger TE, Guidry CA, Rotstein OD, et al. Longer-duration antimicrobial therapy does not prevent treatment failure in high-risk patients with complicated intra-abdominal infections. Surg Infect. 2017;18:659-663.
13. Rattan R, Allen CJ, Sawyer RG, et al. Patients with risk factors for complications do not require longer antimicrobial therapy for complicated intra-abdominal infection. Am Surg. 2016;82:860-866.
14. Montravers P, Tubach F, Lescot T, et al. Short-course antibiotic therapy for critically ill patients treated for postoperative intra-abdominal infection: the DURAPOP randomised clinical trial. Intensive Care Med. 2018;44:300-310.
15. CDC. Antibiotic Resistance Threats in the United States, 2013. Atlanta, GA: CDC; 2013.
16. Kollef MH. Optimizing antibiotic therapy in the intensive care unit setting. Crit Care. 2001;5:189-195.
17. Hanretty AM, Gallagher JC. Shortened course of antibiotics for bacterial infections: a systematic review of randomized controlled trials. Pharmacotherapy. 2018;38:674-687.
To comment on this article, contactrdavidson@uspharmacist.com.





