US Pharm. 2007(32):20-23.
Heartburn, one of the classic
symptoms of gastroesophageal reflux disease (GERD), is a very common
occurrence among the general population. In the United States, approximately
44% of the population experience symptoms of GERD monthly, while 20% have
symptoms on a weekly basis.1 The prevalence of GERD increases with
age in those 40 or older. The increased incidence of GERD in seniors remains
unclear; however, changes in the antireflux mechanism that occur with age
(e.g., less consistent esophageal peristalsis, decreased salivary response to
esophageal acid infusion, etc.) may be responsible.2
Pyrosis (heartburn) and acid
regurgitation (passage of gastric contents into the oropharynx) secondary to
chronic reflux are the classic symptoms of this disorder. Excessive reflux of
acidic gastric contents into the esophagus, resulting in irritation or injury
to the esophageal mucosa, constitutes a diagnosis of GERD. Interestingly,
according to the American College of Gastroenterology guidelines, while the
presence of esophagitis or Barrett's esophagus is diagnostic for GERD, normal
endoscopy results are found in the majority of symptomatic patients and
neither rule out GERD nor indicate a lower severity of symptoms.3
The guidelines further state that such patients with so-called "endoscopic
negative" disease have similar requirements for therapy and should receive the
same treatment considerations as patients who have erosive esophagitis,
including, in some patients, long-term proton pump inhibitor (PPI) therapy.
Success of GERD Treatment
The goals of
therapy for GERD are to: 1) alleviate or eliminate symptoms; 2) decrease
frequency or recurrence and duration of GERD; 3) promote healing of mucosa;
and 4) prevent complications. The PPIs are more effective than histamine2
-receptor antagonists (H2RAs) in achieving these goals of therapy.
They are capable of providing complete resolution of symptoms and healing of
esophagitis. Healing rates after four weeks and eight weeks of therapy are
approximately 80% and 90%, respectively, with PPIs (esomeprazole [Nexium],
lansoprazole [Prevacid], omeprazole [Prilosec], pantoprazole [Protonix],
rabeprazole [Aciphex]) as compared to approximately 50% and 75%, respectively,
with H2RAs (cimetidine [Tagamet], famotidine [Tagamet], nizatidine
[Axid], ranitidine [Zantac]).4
Clinical Features
Often, there is a
lack of heartburn in an elderly patient with GERD. While the severity of
symptoms in this population may be decreased, this does not indicate lack of
esophagitis. In fact, older people are more likely to develop severe disease.
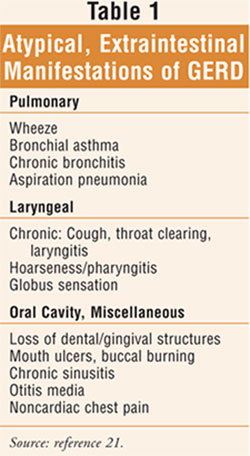
Atypical symptoms may manifest outside the intestinal tract (TABLE 1)
as cough, wheeze associated with asthma, noncardiac chest pain, dental
deterioration, jaw pain, and anemia.5,6 Alarm symptoms of GERD
require immediate medical attention; these include dysphagia, odynophagia,
choking, bleeding, weight loss, and chest pain. GERD is also associated with
specific respiratory conditions including bronchitis, laryngopharyngitis,
refractory asthma, and aspiration pneumonia.7 Furthermore, since
severity of symptoms often does not correlate with the degree of esophagitis,
it has been recommended that all elderly patients experiencing onset of
symptoms be considered for diagnostic endoscopy.2
GERD is often poorly diagnosed
and underreported in the geriatric population since seniors typically present
with atypical symptoms, as noted above. Differentiation from other disease
states such as asthma and angina is required. Acid suppressant therapy is
important in this population since seniors are less likely to report frequent
or severe heartburn secondary to a decline in esophageal sensitivity. In
addition, seniors are at increased risk for GERD-associated bleeding if they
take daily aspirin, antiplatelet agents, anticoagulants, NSAIDs, and selective
serotonin reuptake inhibitors.8 Since the elderly are at higher
risk for infection, are particularly susceptible to the respiratory
complications of GERD, and are a population for whom pneumonia is a major
cause of mortality, the need for acid-suppressant therapy is clear in
appropriate candidates.9
Current Treatment Philosophy
In general, the current treatment
philosophy for acid suppression is divided into phases. Phase I involves
lifestyle modifications (e.g., dietary changes [avoidance of foods that
decrease lower esophageal sphincter pressure or are direct irritants], weight
reduction, smoking cessation, head-of-bed elevation [6-8 inches], avoiding
eating three hours before bedtime, avoiding tight-fitting clothes) and OTC
antacids or low-dose H2RAs.1 Phase II involves the use
of lifestyle modifications, and prescription H2RAs or PPIs.
Finally, phase III involves intervention therapies (e.g., surgery). For
seniors, the step-down approach (starting at phase II) is recommended. This
involves initiating the most effective regimen, which is a full dosage of a
PPI, and, once symptoms are under control, switching to lower doses of the PPI
for maintenance therapy.10 This approach may be more rational,
compared to the step-up (starting at phase I) approach, based on the
evidence showing superior efficacy of PPIs over H2RAs across all
grades of GERD severity. No comparative studies have evaluated whether the
step-down or step-up approach is more cost effective in elderly patients.
10 PPI therapy does not require dosage adjustment for renal
insufficiency, as compared with H2RAs, often an issue in the
elderly.
Refractory Symptoms and
Nocturnal Acid Breakthrough
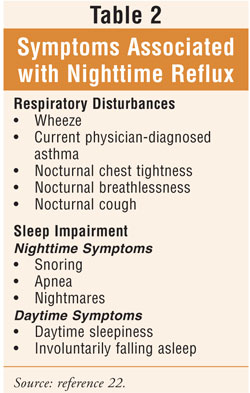
Severe symptoms or
extraintestinal manifestations may require twice-daily PPI dosing, with
two-thirds of patients continuing to make acid, particularly at night. This is
called nocturnal acid breakthrough.4 Clinicians should
become familiar with the symptoms associated with nighttime reflux (TABLE 2
) so that recognition may be facilitated. Refractory symptoms on
twice-daily PPI therapy are often treated with the addition of an H2
RA at night (with short-lived effect secondary to tolerance development).
4,10,11 In the refractory patient, additional testing should be
considered to exclude complications, and the diagnosis may need to be changed.
Since sleep is a significant risk factor for acid migration to the proximal
esophagus and markedly prolongs acid clearance, nighttime reflux may increase
the risk of microaspiration.12 In fact sleep--particularly sound
sleep--may represent risk for adverse physiologic events.13
Apparently Refractory
Reflux Disease: Approach to Therapy
In refractory
patients, it is recommended that the clinician verify adherence with lifestyle
modifications and assess adherence with PPI therapy. Making certain that the
patient is taking the daily PPI dose before the first meal of the day is
essential. Additionally, pharmacotherapy adjustments may need to be made: 1)
use maximum FDA-labeled dosage; 2) increase PPI dose to twice daily (e.g.,
off-label use may be required in some situations) with administration of the
second dose 30 minutes prior to evening meal, not at bedtime; 3) use of
on-demand PPI therapy may be suitable for patients with mild to moderate
symptoms (however, studies are lacking); 4) patients who have tried less
effective medications without success should have access to long-term PPI
therapy; 5) add a prescription-strength H2RA at night; 6) add an
OTC H2RA (e.g., ranitidine 75 mg) 30 to 60 minutes before
eating/drinking items causing heartburn; and, finally, 7) if patient remains
refractory to therapy, refer to specialist.4,10,14 It is important
to bear in mind that acid is usually unlikely to be the cause of symptoms with
twice-daily dosing of a PPI. Response to aggressive acid suppression is often
the most commonly employed initial tool to indicate GERD etiology in a patient
with atypical symptoms.
GERD and the Asthma Patient
GERD has recently
been recognized as a common trigger of asthma, potentially via esophageal
acid-induced reflex bronchoconstriction and microaspiration of acid.15
In addition, allergic rhinitis (AR) coexists with asthma, although it is
unknown whether asthma and AR are different manifestations of an allergic
process or if AR is a discrete asthma trigger.15 In a recent study,
more than 50% of patients with difficult-to-treat asthma were diagnosed with
GERD with evidence indicating the important role of acid reflux in patients
who have problems controlling their asthma.16 This is an important
concept since there are approximately 5,000 deaths in the U.S. per year
attributable to asthma, with most cases classified as preventable with
treatment.15 Furthermore, the prognosis of asthma is good with
adequate access and adherence to treatment; appropriate treatment for acute
exacerbations and chronic asthma is required.
GERD in the asthma patient may
require higher dosages and a longer course of treatment as compared to
patients with classic symptoms.4 Initial empiric therapy with
twice-daily PPIs for two to three months is generally recommended.
Potential Risks with
Acid-Suppressant Therapy
There have been
some concerns and controversies regarding the use of acid-suppressant therapy.
PPIs have been associated with gastric carcinoid tumors observed in rats but
have not been demonstrated in humans.17 According to one
case-controlled study looking at patients older than 50, long-term PPI
therapy, particularly at high doses, is associated with an increased risk of
hip fracture, possibly due to interference with calcium absorption through
induction of hypochlorhydria and may potentially reduce bone resorption.18
Another case-controlled study found support of an association between chronic
use of H2RAs and PPIs by older adults and the development of
vitamin B12 deficiency. While additional studies are needed to
confirm these findings, it is an important consideration as vitamin B12
deficiency is a common problem among the elderly.19 Acid
suppression with H2RAs and PPIs is associated with an increased
risk of community-acquired pneumonia, probably due to a reduction of gastric
acid secretion that facilitates oral infections.20 This finding is
a dose-response effect with the relative risk of pneumonia modest with H2
RAs, greater with PPIs, and greatest with more than once-daily dosing of a PPI.
20 The researchers recommended that immunocompromised, asthmatic, COPD,
pediatric, and elderly patients should be treated only when needed and with
the lowest effective dosage.
Conclusion
For the senior and
senior refractory patient with GERD, careful consideration of acid-suppression
therapy is recommended due to: atypical symptoms, increased risk of infection,
increased risk of mortality secondary to pneumonia, and risk-associated
medications in the regimen. Clinicians, health care administrators, and
providers need to understand the importance of balancing the benefit of
appropriate and adequate PPI therapy with pharmacoeconomic and long-term
safety concerns. In light of the favorable safety profile, ease of
administration, potential risks of therapy, and high rate of effectiveness of
PPI therapy, long-term therapy is beneficial for chronic or complicated GERD,
and higher-than-approved dosages may be appropriate in certain situations.
Furthermore, unnecessary human and health care costs may potentially be
avoided with the appropriate treatment of GERD, a known trigger of asthma.
References
1. Williams DB,
Schade RR. Gastroesophageal reflux disease. In: Dipiro JT, et al, eds.
Pharmacotherapy: A Pathophysiologic Approach. 6th ed. Appleton & Lange,
2005:613-628.
2. Hila A, Castell DO.
Upper gastrointestinal disorders. In: Hazzard WR, Blass JP, Halter JB, et al,
eds. Principles of Geriatric Medicine and Gerontology. 5th ed. New
York: McGraw-Hill, Inc.; 2003:622-626.
3. Updated ACG
Guidelines for Diagnosis and Treatment of GERD. The American College of
Gastroenterology (ACG). Available at: www.acg.gi.org/physicians/guidelines/
GERDTreatment.pdf. Accessed October 1, 2007.
4. Hoogerwerf WA, Pasricha PJ. Pharmacotherapy of gastric acidity, peptic ulcers, and gastroesophageal reflux disease. In: Brunton, et al., eds. Goodman & Gilman's; 2006: 976-978.
5. Greenwald DA. Aging, the gastrointestinal tract, and risk of acid-related disease. Am J Med. 2004;117(suppl 5A):8S-13S.
6. Johnson DA. Gastroesophageal reflux disease in the elderly--a prevalent and severe disease. Rev Gastroenterol Disord. 2004;4(suppl 4):S16-24.
7. Sataloff RT, Castell DO, et al. Reflux Laryngitis and Related Disorders. 2nd ed. New York, NY: Delmar Thomson Learning; 2003.
8. Semla TP, Beizer JL, Higbee MD. Geriatric Dosage Handbook. 12th ed. Hudson, Ohio: Lexi-Comp, Inc.; 2007.
9. Zagaria ME. GERD: Maximizing Outcomes in Special Populations. In: Strategies for Optimal Disease Management in Patients with GERD. Presented in association with the Academy of Managed Care Pharmacists; Hynes Convention Center, Boston Massachusetts; October 25, 2007.
10. Pilotto A, Franceschi M, Paris F. Recent advances in the treatment of GERD in the elderly: focus on proton pump inhibitors. Int J Clin Pract. 2005;59:1204-1209.
11. Winter HS, Gold BD, Nelson SP. Pediatric GERD: a problem-based approach to understanding treatment. 2005. Medscape. Available at: www.medscape.com/viewprogram/4715. Accessed October 22, 2007.
12. Orr WC, Elsenbruch S, Harnish MJ, et al. Proximal migration of esophageal acid perfusions during waking and sleep. Am J Gastroenterol. 2000;95:37-42.
13. Orr WC. Sleep issues in gastroesophageal reflux disease: beyond simple heartburn control. Rev Gastroenterol Disord. 2003;3(suppl 4):S22-S29.
14. Vaezi MF. Atypical manifestations of gastroesophageal reflux disease. Medscape General Medicine. Available at: www.medscape.com/viewarticle/506303_print. Accessed October 1, 2007.
15. Beers MH, Porter RS, Jones TV, et al. The Merck Manual of Diagnosis and Therapy. 18th ed. Whitehouse Station, NJ: Merck Research Laboratories; 2006:112-113.
16. Wong CH, Chua CJ, Liam CK, et al. Gastro-oesophageal reflux disease in ‘difficult-to-control' asthma: prevalence and response to treatment with acid suppressive therapy. Aliment Pharmacol Ther. 2006;23:1321-1327.
17. Howland RD, Mycek MJ. Pharmacology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006:323-329.
18. Yang YX, Lewis JD, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296:2947-2954.
19. Valuck RJ, Rusein JM. A case-controlled study on adverse effects: H2 blocker or proton pump inhibitor use and risk of vitamin B12 deficiency in older adults. J Clin Epidemiol . 2004;57:422-428.
20. Laheij RJ, Sturkenboom MC, Hassing RJ, et al. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA. 2004;292:1955-1960.
21. Hogan WJ, Shaker R. Supraesophageal complications of gastroesophageal reflux. Dis Mon. 2000;46:193-232.
22. Gislason T, Janson C, Vermeire P, et al. Respiratory symptoms and nocturnal gastroesophageal reflux: a population-based study of young adults in three European countries. Chest . 2002;121:158-163.
To comment on this article, contact editor@uspharmacist.com.





