US Pharm. 2018;43(7):6-10.
Vaccines are considered one of the greatest public health interventions of the 20th century. The incidence, prevalence, morbidity, and mortality of many infectious diseases have significantly decreased because of immunization strategies. It is estimated that for every United States birth cohort receiving recommended childhood vaccinations, approximately 20 million illnesses and more than 40,000 deaths are prevented.1 Despite the widespread availability of vaccines, disease outbreaks still occur in the U.S. These resurgences can be attributed to a variety of causes, including refusal to vaccinate, incomplete vaccination, waning immunity, and imported cases.1
Tetanus, diphtheria, and pertussis are vaccine-preventable diseases that continue to cause significant morbidity in those who are infected. Since the 2000s, there has been a steady increase in the number of pertussis cases in the U.S. This development has led to new recommendations for a pertussis booster in teenagers and adults. Regardless of this, rates have continued to increase; in 2012 the U.S. experienced the largest pertussis outbreak since 1955.2
In 2005, the FDA approved two tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccines (Tdap).3 The primary objective of Tdap is to protect immunized adolescents against pertussis. Secondary objectives include reducing the pertussis reservoir within the population and reducing the incidence of pertussis in nonimmunized peers and other age groups, including infants.3 Since the introduction of these vaccines in 2005, Tdap immunization rates for adolescents aged 13 to 17 years have steadily increased to 86.4% in 2015, meeting the Healthy People 2020 target of 80%.4,5 However, only 23% of adults aged 19 years and older have received a dose of Tdap and only 48.8% of pregnant women in the 2015–2016 influenza season were vaccinated.4,6
As easily accessible healthcare providers, pharmacists play a pivotal role in increasing immunization rates and in serving as educators, facilitators, and administrators of vaccines. They are uniquely situated to help expand access to immunizations and specifically address the suboptimal Tdap immunization rates among adults and pregnant women.
Tetanus
Tetanus is an acute and often fatal disease that has afflicted the human population since the 5th century bc. It is caused by a potent endotoxin, tetanospasmin, which is produced by Clostridium tetani. This organism is found in soil and animal excrement and is often transmitted through wounds contaminated with C tetani; about 5% of tetanus cases are a result of chronic skin ulcers. Under anaerobic conditions, such as necrotic tissue, C tetani germinates and the toxin is spread through the blood and lymphatic systems. Tetanospasmin binds irreversibly with neural tissue, causing spasms and rigidity of voluntary muscles, mainly in the jaw (lockjaw) and neck. Tetanus is the only vaccine-preventable disease that is not contagious.7-9
When the vaccine was introduced into the childhood vaccination schedule and national reporting of tetanus began in the late 1940s, the reported number of tetanus cases steadily declined; tetanus cases have decreased by more than 95% and the number of tetanus-related deaths has decreased by more than 99%. Sporadic cases continue to occur, mainly in adults who either were never vaccinated or had not received a booster in the previous 10 years. In a 2014 vaccination-coverage survey, 62.6% of adults aged 19 through 49 years reported receiving a tetanus toxoid–containing vaccine within the last 10 years, compared with 57.7% of adults aged 65 years and older.7,8,10
Diphtheria
Diphtheria is an infectious disease caused by toxigenic strains of the aerobic gram-positive bacterium Corynebacterium diphtheriae; it can affect any mucous membrane. This disease was first described by Hippocrates in the 5th century bc and it has been responsible for both endemic and epidemic diseases throughout history. It is a highly contagious disease that is spread person-to-person by respiratory droplets or direct contact with respiratory secretions, discharges from skin lesions, or, rarely, fomites. Once a patient is infected, the bacteria produce a toxin that causes inflammation, tissue damage, and pseudomembrane formation. Tissue injury allows for the absorption of the toxin into the bloodstream and lymphatic system, distributing it to other bodily tissues and resulting in complications, including damage to the myocardium, nervous system, and kidneys.11-13
Diphtheria was once a major cause of morbidity and mortality in children. In the 1920s, 100,000 to 200,000 cases of diphtheria and 13,000 to 15,000 deaths were reported annually. Widespread use of diphtheria-containing vaccines beginning in the 1920s and the incorporation of these vaccines into the childhood vaccination schedule in the late 1940s have led to the significant reduction in diphtheria incidence in the U.S. From 1996 to 2016, a total of 13 diphtheria cases and three deaths were reported to the CDC.14 Many of the cases occurred in patients who were unimmunized or inadequately immunized.7,11,13
Pertussis
Pertussis, also known as whooping cough, is a respiratory illness that is caused by the gram-negative bacterium Bordatella pertussis. It is a highly contagious illness transmitted through respiratory droplets or secretions via direct contact or sharing of a confined space. Patients are most contagious when respiratory secretions are first produced up until 2 weeks after the onset of cough. Once inhaled, the bacteria attach to the respiratory cilia and produce a toxin that paralyzes the cilia, interfering with the clearance of pulmonary secretions. An exudate forms in the respiratory tract, compromising the small airways, especially in infants. This predisposes the patient to atelectasis, cough, cyanosis, and pneumonia.2,15
Prior to any available vaccine, pertussis was a very common childhood disease associated with significant morbidity and mortality. More than 1 million cases were reported between 1940 and 1945. After the introduction of the first whole-cell pertussis vaccine in the 1940s, the incidence of pertussis gradually began to decline; in 1960, 150,000 cases were reported, and by 1990 fewer than 2,900 cases per year were reported. Although pertussis vaccination has led to a significant decrease in the number of pertussis cases and deaths, pertussis is still a major public health concern.2,15
Over the last few decades there has been an increase in the incidence of pertussis, with significant peaks observed in recent years. In 2012, there were a reported 48,277 cases nationwide, the largest number of cases reported since 1955.2 Since then, the number of cases has waned, but still remains significantly higher than numbers observed in the 1990s and early 2000s.2 Incidence of pertussis is highest among infants; between 2010 and 2015, 80% of all pertussis-related deaths were among those aged 6 months or less.2 The second most highly affected population is school-aged children and adolescents.2 The CDC estimates that up to 10% of all cases of pertussis are recognized.16 Pertussis remains the most commonly reported vaccine-preventable disease in the U.S. among children aged less than 5 years.2,7,15 Vaccination is the best defense against pertussis; however, a large proportion of patients who develop the disease are too young to receive the vaccine.
Vaccines for Prevention
Several vaccine products are available for the prevention of tetanus, diphtheria, and pertussis, each differing in composition and indications (Table 1). It is imperative for healthcare professionals to be aware of these differences, because confusion over these products has led to a significant number of medication errors each year.17 Confusion often occurs because these products contain similar antigenic components but differ in strength and the order in which the components are listed.
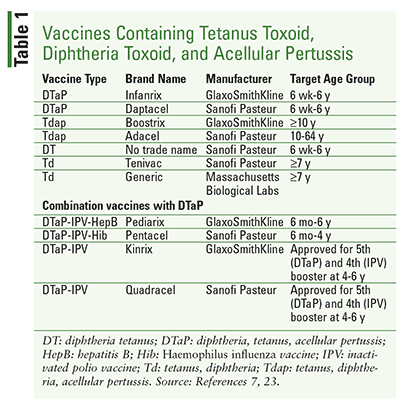
The older formulations are referred to as DTaP (available under the brand names Tripedia, Daptacel, and Infanrix) and are indicated for the primary immunization of infants and children aged 6 weeks to 6 years against all three diseases.7 Products referred to as Tdap (Boostrix and Adacel) also have antigenic components to prevent all three conditions, although the diphtheria toxoid concentration is reduced. The latter products are indicated as a single booster dose for the prevention of these diseases in older children, adolescents, and adults.7 Other recommended uses of Tdap for selected clinical situations will be discussed later.
A combination product (Td) that contains only tetanus and diphtheria toxoids is also available and is recommended to be administered every 10 years as a booster dose in adults who have received their primary immunization against tetanus and diphtheria.7
Efficacy of Tdap
Currently, two commercially available Tdap products are approved for use in adolescents and adults as a single-dose booster vaccine against tetanus, diphtheria, and pertussis. These products, Boostrix (GlaxoSmithKline) and Adacel (Sanofi Pasteur), differ in age indications, antigenic components, and diphtheria toxoid concentrations.
Several studies have been conducted to evaluate the effectiveness of Tdap. The efficacy of both the tetanus and diphtheria toxoid components were based on serologic immune responses and were found to be noninferior to the responses observed in existing Td-containing products.7 Because there is no confirmed laboratory correlate of protection against pertussis, the efficacy of the current Tdap vaccines against the disease was inferred by evaluating the serologic response of infants who were vaccinated with three doses of DTaP. Based upon predefined criteria, a single dose of these products demonstrated an efficacy rate noninferior to that of existing childhood vaccines.7,18
Postmarketing evaluations have been performed to assess the efficacy of Tdap vaccines against pertussis. According to the Advisory Committee on Immunization Practice (ACIP), the efficacy of these vaccines in adolescents who received primary immunization as children ranged from 66% to 78%.7 However, as with DTaP, several studies have suggested that the efficacy of Tdap booster doses wanes over time and with advancing age. One study suggested that Tdap was 73% effective within 1 year of administration but diminished to less than 35% within 4 years.19 Another study confirmed the waning efficacy of Tdap and indicated that the effectiveness in preventing pertussis may drop below 10% after 4 or more years postvaccination.20
Safety of Tdap
According to data derived from clinical trials as well as postmarketing surveillance, the commercially available Tdap vaccines are considered safe products. Local reactions such as pain, redness, and swelling at the injection site are the most commonly encountered complaints. Less commonly reported reactions include constitutional symptoms such as headache and fatigue.21,22 There have been no reports to suggest any correlation between the use of Tdap and such rare events as encephalopathy, paralytic seizures, or Guillain-Barré syndrome.7
Contraindications
There are few reasons not to provide the Tdap vaccine to eligible adolescents and adults. If a patient experienced a severe allergic reaction (i.e., anaphylaxis) after a previous dose of any tetanus toxoid, diphtheria, or pertussis-containing vaccine, the use of Tdap is contraindicated. The Tdap vaccine is also contraindicated in patients with a history of encephalopathy not attributable to any identifiable cause within 7 days of administration of a previous pertussis antigen–containing vaccine. These patients should receive the diphtheria toxoid vaccine and avoid any vaccines containing the pertussis toxoid.21,22
Current Indications for Tdap Vaccine
The ACIP has recently updated its recommendations for the use of vaccines for the prevention of tetanus, diphtheria, and pertussis.7 Although practitioners should always consult current ACIP guidelines for updated recommendations concerning the use of vaccines, particularly in special populations, the general indications for the use of Tdap are as follows7:
• Commercially available Tdap vaccines should be used as a single booster dose for all adolescents aged 11 through 18 years who have completed their primary childhood vaccination series with DTaP. This dose should preferably be administered at a preventive-care visit at age 11 to 12 years
• All adults aged 19 years and older who have never received a dose of Tdap should receive a single Tdap dose in place of a decennial Td booster dose (when administered to adults aged >65 years, Boostrix is preferred over Adacel because it has the FDA-approved indication, although both products are considered immunogenic and would provide protection)
• A single dose of Tdap should be administered between 27 and 36 weeks' gestation for all pregnant women during each pregnancy to maximize passive antibody transfer to the infant
• Persons aged 7 to 18 years who are not fully immunized with DTaP should receive a single dose of Tdap as one dose of the catch-up series. These individuals should receive an additional Tdap dose administered at age 11 to 12 years
• Persons 18 years of age and older who have never received their primary immunization for these diseases should receive a series of three vaccinations containing tetanus and diphtheria toxoids, including one dose of Tdap
According to the current guidelines, when indicated, Tdap should be administered to a patient regardless of the time interval since the patient last received a Td-containing product.
Role of the Pharmacist
All adolescents and adults should receive one dose of the Tdap vaccine. Currently, not even half of the adult population has received this vaccine; maintaining high immunization rates is imperative to sustain low incidence levels of vaccine-preventable diseases such as pertussis. Pharmacy-based immunization initiatives have been shown to significantly improve vaccination rates. Pharmacists are pivotal in achieving improved immunization rates by educating patients about the importance and safety of vaccinations and by increasing access to immunization services.
Patient Information
What are the symptoms?
Tetanus can cause your muscles to feel stiff and to spasm. It can cause the muscles in your head to tighten. If this happens, you may not be able to open your mouth, swallow, or breathe.
Diphtheria can cause a thick covering in the back of your throat that can lead to breathing problems.
Pertussis, also known as whooping cough, causes severe coughing, which can lead to breathing problems and disturbed sleep. It can be deadly, especially in young infants.
Who should receive the Tdap vaccine?
The Tdap vaccine is routinely given to children aged 11 to 12 years. People who did not receive Tdap at this age should receive it as soon as possible.
Tdap is also very important for anyone who is aged 65 years or older and for anyone who will be in close contact with a newborn baby. If you have never had a dose of Tdap and are part of this patient population, you should receive one as soon as possible.
Pregnant women should receive a dose of Tdap during every pregnancy. The Tdap vaccine should be given anytime between the 27th and 36th weeks of pregnancy.
Do I need to receive the Tdap vaccine every 10 years?
You need only one dose of the Tdap vaccine. After that, you should continue to get vaccinated against diphtheria and tetanus every 10 years.
Are there any side effects to the Tdap vaccine?
Most people do not have any problems after receiving the vaccine. Some common side effects that you may experience include redness, swelling, and soreness at the injection site, headache, tiredness, and mild fever.
More serious side effects are rare. If you have ever had a severe allergic reaction to latex or a previous dose of a vaccine, let your healthcare provider know.
Where can I receive more information about vaccines?
You can speak with your pharmacist, doctor, or other healthcare provider. You can also contact your local or state health department or call the CDC at 1-800-232-4636 (1-800-CDC-INFO) or visit the CDC's website at: www.cdc.gov/vaccines.
REFERENCES
1. Ventola CL. Immunization in the United States: recommendations, barriers, and measures to improve compliance. Pharm Ther. 2016;41(7):426-436.
2. CDC. Surveillance manual. Vaccine preventable diseases. Pertussis. May 21, 2018. www.cec.gov/vaccines/pubs/survmanual/chpt10-pertussis.html. Accessed June 8, 2018.
3. American Academy of Pediatrics Committee on Infectious Diseases. Prevention of pertussis among adolescents: recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine. Pediatrics. 2006;117(3):965-978.
4. Williams WW. Surveillance of vaccination coverage among adult populations—United States, 2015. MMWR Surveill Summ. 2017;66.
5. U.S. Department of Health and Human Services. Office of Disease Prevention and Health Promotion. HealthyPeople. 2020 topics and objectives. Immunization and infectious disease. www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives. Accessed June 10, 2018.
6. CDC. Toolkit for prenatal care providers. Pregnancy and vaccination. Maternal vaccination coverage. December 6, 2017. www.cdc.gov/vaccines/pregnancy/hcp-toolkit/maternal-vaccination-coverage.html. Accessed June 10, 2018.
7. Liang JL, Tiwari T, Moro P, et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2018;67(2):1-44.
8. Tetanus: background, pathophysiology, etiology. March 2018. https://emedicine.medscape.com/article/229594-overview#a2. Accessed June 8, 2018.
9. CDC. The Pink Book. Epidemiology of vaccine preventable diseases. Tetanus. May 16, 2018. www.cdc.gov/vaccines/pubs/pinkbook/tetanus.html. Accessed June 8, 2018.
10. Williams WW. Surveillance of vaccination coverage among adult populations—United States, 2014. MMWR Surveill Summ. 2016;65.
11. CDC. The Pink Book. Epidemiology of vaccine preventable diseases. Diphtheria. May 16, 2018. www.cdc.gov/vaccines/pubs/pinkbook/dip.html. Accessed June 8, 2018.
12. Diphtheria: background, pathophysiology, epidemiology. May 2018. https://emedicine.medscape.com/article/782051-overview. Accessed June 8, 2018.
13. CDC. Surveillance manual. Vaccine preventable diseases. Diphtheria. March 29, 2018. www.cdc.gov/vaccines/pubs/surv-manual/chpt01-dip.html. Accessed June 8, 2018.
14. CDC. The Pink Book. Epidemiology of vaccine preventable diseases. Data and statistics. May 16, 2018. www.cdc.gov/vaccines/pubs/pinkbook/appendix/appdx-e.html. Accessed June 8, 2018.
15. CDC. The Pink Book. Epidemiology of vaccine preventable diseases. Pertussis. May 21, 2018. www.cdc.gov/vaccines/pubs/pinkbook/pert.html. Accessed June 8, 2018.
16. Bocka JJ, McNeil BK. Pertussis: practice essentials, background, etiology and pathophysiology. May 2018. https://emedicine.medscape.com/article/967268-overview. Accessed June 8, 2018.
17. Institute for Safe Medication Practices. DTaP-Tdap mix-ups now affecting hundreds of patients. www.ismp.org/resources/dtap-tdap-mix-ups-now-affecting-hundreds-patients. Accessed June 8, 2018.
18. Plosker GL. Combined, reduced-antigen content tetanus, diphtheria, and acellular pertussis vaccine (Boostrix): a review of its use as a single-dose booster immunization in individuals aged 10-64 years in the US. BioDrugs Clin Immunother Biopharm Gene Ther. 2009;23(4):253-267.
19. Acosta AM, DeBolt C, Tasslimi A, et al. Tdap vaccine effectiveness in adolescents during the 2012 Washington State pertussis epidemic. Pediatrics. 2015;135(6):981-989.
20. Klein NP, Bartlett J, Fireman B, Baxter R. Waning Tdap effectiveness in adolescents. 2016;137(3):e20153326. Epub February 5, 2016.
21. Adacel (diphtheria, tetanus, and acellular pertussis adult vaccine [Tdap]) package insert. Swiftwater, PA: Sanofi Pasteur, Inc; 2017.
22. Boostrix (tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine [Tdap]) package insert. Research Triangle Park, NC: GlaxoSmithKline; 2016.
23. CDC. U.S. vaccine names. December 27, 2017. www.cdc.gov/vaccines/terms/usvaccines.html. Accessed June 10, 2018.
To comment on this article, contact rdavidson@uspharmacist.com.






