US Pharm. 2013;38(9):64-68.
More than 347 million people worldwide and over 26 million people in the United States have been diagnosed with diabetes mellitus.1 Type 2 diabetes accounts for more than 90% of all diagnosed cases. Additionally, the incidence of new cases is steadily increasing with an estimated 54 million Americans characterized as having prediabetes. A contributing factor to an increase in the number of new cases may be associated with childhood obesity. As a result, the cost of treatment in 2007 was estimated at 174 billion dollars and is speculated to increase in the years to come.2

Diabetes Management
If left untreated, patients will maintain an elevated blood glucose level (hyperglycemia) though the body starves for energy. The main symptoms of hyperglycemia include polyuria, polydipsia, polyphagia, and fatigue. Complications of diabetes may be microvascular (i.e., retinopathy, nephropathy, peripheral neuropathy, and autonomic neuropathy) or macrovascular (i.e., cardiovascular disease, cerebrovascular disease, peripheral artery disease). As such, it is important for individuals to start lifestyle modifications and pharmacologic therapy upon diagnosis. Though therapy is typically evaluated using daily glucose monitoring and hemoglobin A1C levels, recent advances in technology have led to the development of continuous glucose monitoring devices, which afford the ability to better visualize fluctuations in daily glucose levels. The purpose of this article is to discuss Dexcom’s newest continuous glucose monitor, the G4 Platinum, and its possible implications in the management of diabetes.
G4 Platinum Continuous Glucose Monitor
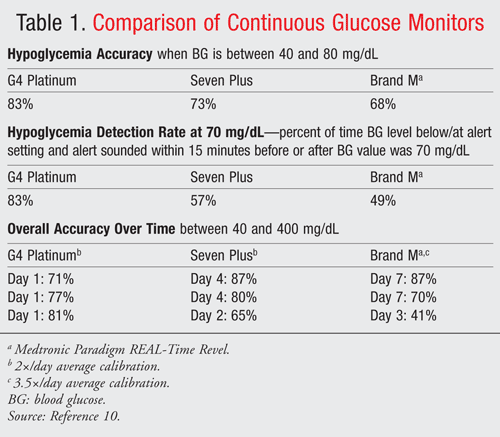
The G4 Platinum glucose monitor is an advancement to Dexcom’s Seven Plus device. The G4 Platinum consists of three parts (sensor, transmitter, and receiver), which will be discussed in greater detail later in this article.3 Available only by prescription, this device has new features that include longer transmission range, color LCD display screen, customizable glucose alerts, and a slimmer, more discrete design. Used as adjunct therapy, the G4 Platinum is indicated for individuals 18 years of age or older and is covered by most insurance companies for patients who have been prescribed insulin. This device measures an individual’s glucose readings every 5 minutes for 7 days and appears to be ideal for patients who experience frequent fluctuations in glucose levels. Dexcom provided initial findings that indicated “approximately 19% improvement in overall accuracy for the Dexcom G4 Platinum compared to the Seven Plus, and approximately a 30% improvement in accuracy in the hypoglycemia range (i.e., when blood glucose level is less than 70 mg/dL).”4 A comparison of continuous glucose monitors is shown in TABLE 1.
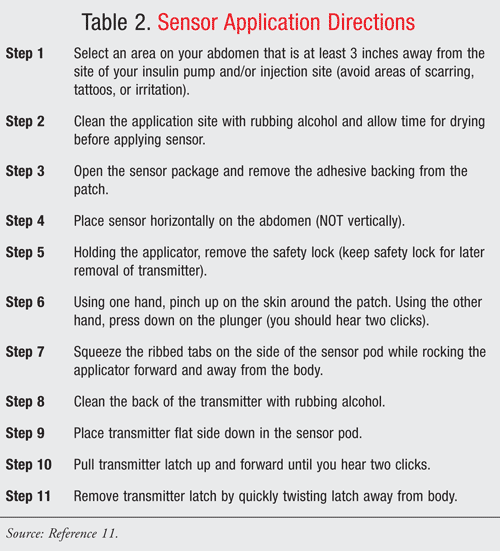
G4 Platinum Sensor: The sensor is a fine, hair-like needle that is inserted into the patient’s abdominal region, similar to an insulin pump. The needle is inserted using a detachable plunger and held in place with a circular adhesive patch for 7 days. Sensor durability surpasses that of the Seven Plus with a decreased incidence of fracture when exposed to repetitive bending and resistance. In the event the sensor breaks under the skin or a problem occurs, the patient should contact his or her prescriber and call Dexcom’s technical support. Step-by-step sensor application instructions are shown in TABLE 2 .
G4 Platinum Transmitter: The transmitter is a grey chip that snaps into the sensor pod. It is responsible for relaying glucose readings to the receiver every 5 minutes through wireless data transmission. While the sensors are only good for 7 days, the life of the transmitter is approximately 6 months and should not be discarded after initial use. Should a low battery indicator light appear, patients should order a new transmitter. Each transmitter comes with its own specific ID code, which must be synchronized with the receiver before it can be used.
G4 Platinum Receiver: The receiver is a small device similar in size to a standard smartphone. The receiver has an LCD color screen that displays current glucose readings, battery life, time frame, and glucose trends. Each dot that appears on the screen represents a specific glucose reading. Dots that appear red or yellow indicate hypoglycemic or hyperglycemic measurements, respectively. A unique feature of this model is a greater receiver transmitter range, giving patients more freedom and mobility. Compared to other continuous glucose monitoring devices, this model requires less calibration (every 12 hours) in addition to the inclusion of a standard feature: a nonchangeable hypoglycemic alert that is set at 55 mg/dL. Blood glucose trends may also be recorded by this device.
Clinical Efficacy
Several clinical studies have been completed demonstrating the efficacy of continuous glucose monitoring in the management of diabetes.5-7 Vigersky et al. demonstrated the benefits of using continuous blood glucose monitoring in a randomized, controlled trial with patients using the Seven Series Dexcom model. This trial compared diet and exercise alone to other glucose-lowering therapies (except prandial insulin). At the end of the 52-week study, results indicated that self-monitoring of blood glucose along with the Seven Series was more effective in lowering hemoglobin A1C and regulating blood glucose levels.8 A new clinical trial is being conducted comparing the accuracy of the new Dexcom G4 Platinum and Medtronic Enlite System in patients with type 1 diabetes. This trial should be completed in January 2014.9
Conclusion
The Dexcom G4 Platinum allows patients to continuously monitor their blood glucose throughout the day for up to 7 days. New features include improved accuracy and monitoring continuous glucose fluctuations, affording patients to be more active with their diabetes management. For more information on the G4 Platinum, visit www.dexcom.com or call 1-858-200-0200.
REFERENCES
1. World Health Organization, Diabetes Fact Sheet, 2012.
www.who.int/mediacentre/factsheets/fs312/en/index.html. Accessed
February 4, 2013.
2. U.S. Department of Human Health
Services, Centers for Disease Control and Prevention, 2011. National
diabetes fact sheet national estimates and general information on
diabetes and prediabetes in the United States, 2011.
www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed February 2, 2013.
3. Dexcom G4 PLATINUM User Guide, 2012.
4. Dexcom. U.S. FDA Approves the Dexcom G4™ PLATINUM Continuous Glucose Monitor (CGM). http://files.shareholder.com/downloads/DXCM/2303969857x0x603924/e6216098-1d6b-45b4-b4ed-1aa03740e97f/DXCM_News_2012_10_8_General.pdf.
Accessed February 4, 2013.
5. Tamborlane W, Beck R, Bode B, et al.
Juvenile Diabetes Research Foundation Continuous Glucose Monitoring
Study Group. Continuous glucose monitoring and intensive treatment of
type 1 diabetes. N Engl J Med. 2008;359:1464-1476.
6. Deiss D, Bolinder J, Riveline J, et
al. Improved glycemic control in poorly controlled patients with type 1
diabetes using real-time continuous glucose monitoring. Diabetes Care. 2006;29:2730-2732.
7. Zick R, Petersen B, Richter M, Haug C. SAFIR Study
Group. Comparison of continuous blood glucose measurement with
conventional documentation of hypoglycemia in patients with type 2
diabetes on multiple daily insulin injection therapy. Diabetes Technol Ther. 2007;9:483-492.
8. Vigersky R, Fonda S, Chellappa M, et
al. Short-and long-term effects of real-time continuous glucose
monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35:32-38.
9. U.S. National Institutes of Health,
Clinical Trials.gov. Standardized Procedure for the Assessment of
New-to-market Continuous Glucose Monitoring Systems (SPACE2).
www.clinical
trials.gov/ct2/show/NCT01751932?term=dexcom+g4+platinum &rank=1. Accessed February 2, 2013.
10. Dexcom.com. Professionals;
Comparison of ontinuous Blood Glucose Monitors.
www.dexcom.com/healthcare-professionals/hypoglycemia. Accessed February
2, 2013.
11. Dexcom.com. Quick Start Guide. www.dexcom.com/sites/dexcom.com/files/professionals/education/Quick_Start_Guide_G4_USLBL010799Rev10.pdf. Accessed February 1, 2013.
To comment on this article, contact rdavidson@uspharmacist.com.





