US Pharm. 2022;47(10):18-22.
Type 2 diabetes mellitus (T2DM) is largely attributed to insulin resistance, secondary to reduced beta-cell functioning in the pancreas.1 Also known as the “ominous octet,” the pathophysiology of T2DM has been recognized as much more complex, impacting not only the pancreas but also the muscle, liver, adipocytes, gastrointestinal tract, alpha-cells, kidney, and brain.2 Thus, T2DM pharmacotherapy sets out to target these various pathways to reduce hyperglycemia.
Incretin hormones inherently work by being released in the gastrointestinal (GI) tract in response to glucose consumption, thereby increasing insulin secretion by the pancreas and decreasing glucagon production, ultimately leading to lower sugars.3,4 Referred to as the incretin effect, they exert their effects by binding to their respective receptors of the pancreas’ beta cells to ultimately release insulin. Glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are the two incretins that have been identified as primarily responsible for postprandial insulin secretion, with GIP being the predominant incretin to release insulin in response to glucose intake.4 The GIP hormone acts on the pancreas, bone, fat, GI tract, heart, and brain, whereas GLP-1 acts on the pancreas, GI tract, kidney, heart, and brain.3 The GLP-1 incretin promotes satiety, slows gastric emptying, decreases glucagon secretion, and decreases food intake, resulting in weight loss. GIP’s effects on gastric emptying are not entirely clear, but coactivation of the GIP and GLP-1 receptors seems to produce an additive effect when insulin is secreted. In patients with T2DM, the incretin effect is diminished.
The first-in-class drug tirzepatide was approved by the FDA in May 2022 for the adjunctive treatment of T2DM. Also nicknamed “twincretin,” tirzepatide is a dual GIP/GLP-1 receptor agonist (GLP-1 RA) that works by activating the GIP and GLP-1 receptors, enhancing insulin secretion in response to glucose intake, decreasing glucagon levels, improving insulin sensitivity, decreasing food intake, and slowing gastric emptying.5 The delay is largest after the initial dose, or the first dose of a dose increase, but the effect diminishes over time.
Clinical Efficacy
The outcomes of five phase III clinical trials, part of the SURPASS program, informed the FDA’s approval of tirzepatide in adults with T2DM. Each trial compared tirzepatide 5 mg, 10 mg, and 15 mg to either placebo, semaglutide, or insulin, with or without other background antihyperglycemics. The primary endpoint for each trial was change in hemoglobin A1c (HbA1c) from baseline to the end of the trial. Secondary efficacy endpoints included but were not limited to change in body weight; percentage of patients achieving HbA1c <7%, <6.5%, and <5.7%; impact on serum lipids; and impact on blood pressures. TABLE 1 summarizes the efficacy of tirzepatide and the respective comparator groups in each clinical trial.
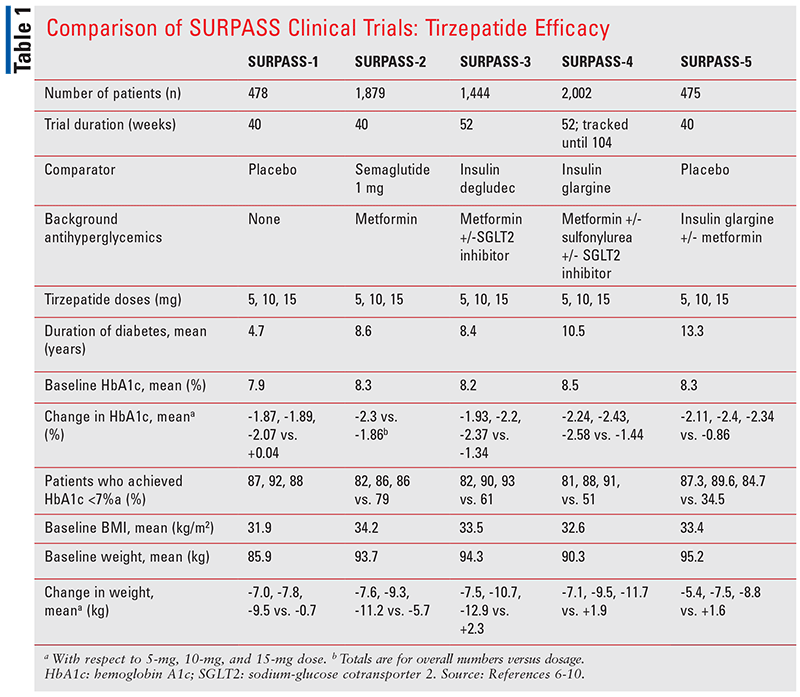
SURPASS-1 compared tirzepatide’s different doses to placebo and demonstrated superiority in HbA1c reduction with each dose comparison. The mean treatment differences compared with placebo were also statistically significant (P <.0001), where 87% to 92% of patients achieved an HbA1c <7% compared with placebo.6 The mean HbA1c concentrations by the end of the study were close to or less than 6% for each dose of tirzepatide.6
In SURPASS-2, tirzepatide was compared with once-weekly GLP-1 RA semaglutide, demonstrating both noninferiority and superiority to it.7 More patients consistently achieved HbA1c <7%, <6.5%, and <5.7% in the tirzepatide group compared with the semaglutide group.7 Differences in weight loss were also statistically significant and superior (P <.001 for each comparison) when comparing each tirzepatide dose with semaglutide.7
Tirzepatide 10 mg and 15 mg were noted to be both noninferior and superior to once-daily insulin degludec in SURPASS-3 when added to metformin, with or without sodium-glucose cotransporter 2 (SGLT2) inhibitors (P <.0001).8 By the end of the trial, patients in the comparator group were using an average of approximately 49 units of insulin degludec.8 Regardless of whether a patient had an SGLT2 inhibitor added to background metformin (32% of trial participants were taking both an SGLT2 inhibitor and metformin), no differences in HbA1c reduction were seen.8 Notably, 82% to 93% of patients achieved an HbA1c <7% in the tirzepatide treatment group compared with 61% in the insulin group.8 More patients (25.7%) in the insulin group achieved a mean fasting blood glucose level <90 mg/dL compared with patients (6.9%-16.3%) in the tirzepatide group.8 On the other hand, the tirzepatide treatment groups had significantly greater reductions from baseline in their daily self-monitoring blood glucose (SMBG) levels, including the 2-hour postprandial levels.8 Lastly, weight reductions were statistically significant in the tirzepatide groups versus the insulin group.8
The fourth SURPASS trial compared tirzepatide to insulin glargine in patients with increased cardiovascular (CV) risk.9 This study was the longest in duration of the SURPASS trials thus far, lasting a median of 85 weeks, but study endpoints were assessed in terms of changes from baseline to 52 weeks. Tirzepatide 10 mg and 15 mg achieved both noninferiority and superiority compared with insulin glargine in HbA1c reduction; tirzepatide 5 mg was also superior to insulin glargine (P <.0001).9 The HbA1c reduction was sustained as far out as 78 weeks (58% of patients) and 104 weeks (10% of patients).9 Each tirzepatide dose demonstrated greater reductions in daily SMBGs from baseline.9 The mean dose of insulin was 43.5 units by 52 weeks and 47 units by 104 weeks.9 Differences in weight reductions of tirzepatide were superior to insulin; the weight loss was maintained at 104 weeks.9
SURPASS-5 compared tirzepatide against placebo in patients using insulin glargine with or without metformin for 40 weeks. The mean change in HbA1c was greater in the tirzepatide treatment groups compared with the placebo group.10 Fasting glucose numbers were also significantly reduced compared with placebo (P <.001).10 The mean differences in weight loss were also observed to be statistically significant (P <.001).10 By Week 40, the mean changes from baseline in insulin dose for tirzepatide 5 mg, 10 mg, and 15 mg were 13.0%, 8.1%, and -11.4%, respectively, versus 75.0% for placebo (P <.001 for each treatment difference).10
The SURPASS-6 trial compares tirzepatide with insulin lispro three times daily in patients using insulin glargine, with or without metformin; this study is expected to be completed in fall 2022.11 So far, all of these trials have demonstrated tirzepatide’s efficacy and safety in adult patients with T2DM. Earlier this year, the SURPASS-PEDS trial began to assess its effect in children aged 10 to 17 years with T2DM who are using metformin and/or basal insulin, and it is estimated to end in 2027.12
As some GLP-1 RAs have been approved for the management of obesity—regardless of a T2DM diagnosis—the SURMOUNT studies were developed to assess tirzepatide’s efficacy and safety in obesity. By completion, the SURMOUNT-1 studied 2,539 patients who exhibited obesity and at least one associated comorbidity, except T2DM.13 After 72 weeks, patients using tirzepatide 5 mg, 10 mg, and 15 mg experienced a weight loss of 15%, 19.5%, and 20.9%, respectively, compared with 3.1% in patients using placebo. The weight loss differences of each tirzepatide dose were superior compared with placebo (P <.001).13 Significant secondary endpoints included weight reduction of at least 20%; a little more than 50% of patients using tirzepatide 10 mg or 15 mg achieved this goal versus 3% of patients using placebo.13
The SURMOUNT-2 study follows the same methodology as SURMOUNT-1, but it compares tirzepatide 10 mg and 15 mg with placebo in patients with T2DM who have obesity or are overweight.14 The SURMOUNT-3 trial compares tirzepatide to placebo in patients without T2DM but who have obesity or are overweight with associated comorbidities.15 After 36 weeks of using tirzepatide, patients will be randomly assigned to continue treatment or start using a placebo in the SURMOUNT-4 study, which will seek to determine whether weight loss is maintained for a longer period.16 These remaining SURMOUNT studies are estimated to be completed by spring 2023.
Clinical Safety
Common safety endpoints across the SURPASS trials included adverse events, hypersensitivity reactions, mean changes in pulse, systolic and diastolic blood pressures, and hypoglycemia. Tirzepatide was not studied in patients with a history of pancreatitis or gastroparesis, so it is not known what its effect is on patients exhibiting these conditions.
As with other existing GLP-1 RA agents, major adverse effects in each SURPASS trial were predominantly GI-related, including nausea, vomiting, diarrhea, and decreased appetite.6-10 GI events were greater with higher doses of tirzepatide, but they were generally mild to moderate in severity and transient; they occurred mostly during dose escalations. Therefore, patients must start at the lowest dose to ensure tolerance before increasing it to an effective level. Hypoglycemia, injection site reactions, hypersensitivity reactions, and pancreatitis occurred as well, but these were much less common across trials. TABLE 2 summarizes common safety endpoints of each trial.
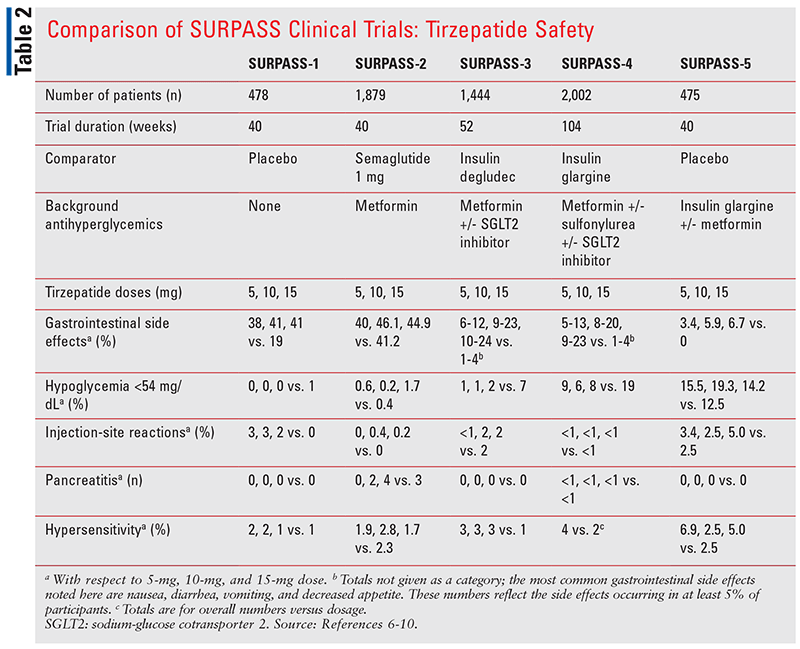
SURPASS-4 also addressed CV safety via the composite endpoint of major adverse cardiovascular events (MACE), discussed further below.
Impact on CV Disease
Across all SURPASS trials, transient increases in heart rate were observed, ranging from 1 to 5 beats per minute more by a study’s end; heart rates returned to baseline after drug discontinuation.6-10 Decreases in mean systolic and diastolic blood pressures were seen across all trials for tirzepatide.6-10 Tirzepatide also demonstrated reductions in lipids in each SURPASS trial: triglycerides decreased, HDL increased, and LDL decreased.6-10
SURPASS-4 was the first of the SURPASS trials to assess tirzepatide’s impact in patients at elevated CV risk; 87% of study patients had a history of CV disease.9 One hundred and nine study patients experienced at least one component of the MACE endpoint, with 5% in the tirzepatide group and 6% in the insulin group; investigators determined that there was no increased risk for MACE in the tirzepatide group versus the insulin group.9
Additional studies regarding tirzepatide’s potential impact on CV outcomes are ongoing. The SURPASS-CVOT trial is studying tirzepatide’s effect on CV events compared with dulaglutide and is expected to be completed in 2024.17 Additionally, the SUMMIT trial is studying the drug’s efficacy and safety against placebo in patients with heart failure with preserved ejection fraction and obesity.18
Additional Warnings and Precautions
Although not studied in humans, tirzepatide is contraindicated in individuals with a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2, as it was found to develop tumors in rats.19 Moreover, although acute pancreatitis and gallbladder disease secondary to tirzepatide were rare in the SURPASS trials, patients should be monitored for signs and symptoms of these issues.6-10,19
Since tirzepatide is known to cause GI adverse effects, dehydration is a possible outcome and can subsequently cause acute kidney injury. When adding tirzepatide to existing antihyperglycemics like meglitinides, insulin, or sulfonylureas, doses of these drugs may need to be reduced to avoid hypoglycemia. Given tirzepatide’s significant effect on the GI tract resulting in delayed gastric emptying, oral contraceptive efficacy may be reduced.19 Gastric emptying is greatest after the first dose, so if patients choose to remain on oral contraceptives, it is recommended that a barrier method be used in the first month of treatment and during the first 4 weeks of any subsequent dose increase.
When and How to Use Tirzepatide
According to the American Diabetes Association’s Standards of Medical Care in Diabetes, selecting the first medication to treat a patient’s T2DM is based on a number of variables, including comorbidities, patient-specific factors, and management needs.20 Historically, the first-line agent has been metformin in adjunct to diet and exercise. However, updated standards indicate that other medications, including GLP-1 RAs, may be used as first-line therapy in patients at high risk for atherosclerotic cardiovascular disease (ASCVD) and/or chronic kidney disease.20 Moreover, if patients have compelling indications that include established ASCVD or kidney disease, a GLP-1 RA that exhibits CV benefits is recommended as one of the treatments.20
When selecting any GLP-1–containing agent, two clinical factors that typically come to mind are HbA1c reduction and weight loss. From a patient’s perspective, side effects and route of administration may also be prioritized. If a patient is already on a GLP-1 RA, adding tirzepatide would be considered a duplication of therapy. If tirzepatide is preferred, then the existing GLP-1 RA would have to be discontinued. As with most existing GLP-1 RAs, tirzepatide is administered as a once-weekly SC injection. It comes as a 0.5-mL prefilled, single-dose autoinjection pen with a hidden needle. Patients start at 2.5 mg once weekly for the first 4 weeks and increase by 2.5 mg every 4 weeks to achieve targeted goals; the maximum available dosage is 15 mg. No dose adjustments are necessary for patients who have renal or hepatic impairment. Tirzepatide is stored in the refrigerator before first use and can be kept out at room temperature for up to 3 weeks. After use, the pen should be disposed of in an appropriate sharps container.
The cost of 4 weeks of tirzepatide is close to $1,000 without insurance.21 The manufacturer provides a copay card for privately insured patients to pay as little as $25 per month.22 Ineligible patients include those who have government-funded insurance, such as Medicare or Medicaid. At the time of this writing, a patient-assistance program does not appear to be available for patients without insurance.
Conclusion
In the growing landscape of T2DM pharmacotherapy, tirzepatide has been proven as a safe and effective agent acting on the GIP/GLP-1 receptors to reduce hyperglycemia. Tirzepatide consistently demonstrated efficacy in reducing HbA1c when compared with the trial’s corresponding comparator arm. In all of the clinical trials, weight reduction was dose-dependent: the higher the dose, the more weight loss. Data from the SURMOUNT-1 study shows significant promise for tirzepatide as yet another option to treat patients with obesity or who are overweight, regardless of T2DM status.
REFERENCES
1. American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes–2022. Diabetes Care. 2022;45(suppl 1):S17-S38.
2. DeFronzo RA, Eldor R, Abdul-Ghani M. Pathophysiologic approach to therapy in patients with newly diagnosed type 2 diabetes. Diabetes Care. 2013;36(suppl 2):S127-S138.
3. Pelle MC, Provenzano M, Zaffina I, et al. Role of a dual glucose-dependent insulinotropic peptide (GIP)/glucagon-like peptide-1 receptor agonist (twincretin) in glycemic control: from pathophysiology to treatment. Life (Basel). 2021;12(1):29.
4. Seino Y, Fukushima M, Yabe D. GIP and GLP-1, the two incretin hormones: similarities and differences. J Diabetes Investig. 2010;1(1-2):8-23.
5. Gasbjerg LS, Helsted MM, Hartmann B, et al. Separate and cobined glucometabolic effects of endogenous glucose-dependent insulinotropic polypeptide and glucagon-like peptide 1 in healthy individuals. Diabetes. 2019;68(5):906-917.
6. Rosenstock J, Wysham C, Frías JP, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021;398(10295):143-155.
7. Frías JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503-515.
8. Ludvik B, Giorgino F, Jódar E, et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet. 2021;398(10300):583-598.
9. Del Prato S, Kahn SE, Pavo I, et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet. 2021;398(10313):1811-1824.
10. Dahl D, Onishi Y, Norwood P, et al. Effect of subcutaneous tirzepatide vs placebo added to titrated insulin glargine on glycemic control in patients with type 2 diabetes: the SURPASS-5 randomized clinical trial. JAMA. 2022;327(6):534-545.
11. Clinicaltrials.gov. A randomized, phase 3, open-label trial comparing the effect of the addition of tirzepatide once weekly versus insulin lispro (U100) three times daily in participants with type 2 diabetes inadequately controlled on insulin glargine (U100) with or without metformin (SURPASS-6). October 19, 2021. www.clinicaltrials.gov/ct2/show/NCT04537923. Accessed July 28, 2022.
12. Clinicaltrials.gov. A randomized, double-blind, placebo-controlled, phase 3 study with an open-label extension assessing the efficacy, safety, and pharmacokinetics/pharmacodynamics of tirzepatide in pediatric and adolescent participants with type 2 diabetes mellitus inadequately controlled with metformin, or basal insulin, or both. September 9, 2022. www.clinicaltrials.gov/ct2/show/NCT05260021. Accessed July 28, 2022.
13. Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205-216.
14. Clinicaltrials.gov. Efficacy and safety of tirzepatide once weekly in participants with type 2 diabetes who have obesity or are overweight: a randomized, double-blind, placebo-controlled trial (SURMOUNT-2). April 19, 2022. www.clinicaltrials.gov/ct2/show/NCT04657003. Accessed July 28, 2022.
15. Clinicaltrials.gov. Efficacy and safety of tirzepatide once weekly versus placebo after an intensive lifestyle program in participants without type 2 diabetes who have obesity or are overweight with weight-related comorbidities: a randomized, double blind, placebo-controlled trial (SURMOUNT-3). March 15, 2022. www.clinicaltrials.gov/ct2/show/study/NCT04657016. Accessed July 31, 2022.
16. Clinicaltrials.gov. Efficacy and safety of tirzepatide once weekly versus placebo for maintenance of weight loss in participants without type 2 diabetes who have obesity or are overweight with weight-related comorbidities: a randomized, double-blind, placebo-controlled trial (SURMOUNT-4). October 1, 2021. www.clinicaltrials.gov/ct2/show/NCT04660643. Accessed July 28, 2022.
17. Clinicaltrials.gov. A randomized, phase 3, open-label trial comparing the effect of the addition of tirzepatide once weekly versus insulin lispro (U100) three times daily in participants with type 2 diabetes inadequately controlled on insulin glargine (U100) with or without metformin (SURPASS-6). October 19, 2021. www.clinicaltrials.gov/ct2/show/NCT04537923. Accessed July 28, 2022.
18. Clinicaltrials.gov. A randomized, double-blind, placebo-controlled, phase 3 study comparing the efficacy and safety of tirzepatide versus placebo in patients with heart failure with preserved ejection fraction and obesity (SUMMIT). September 9, 2022. www.clinicaltrials.gov/ct2/show/NCT04847557. Accessed July 28, 2022.
19. Mounjaro (tirzepatide) package insert. Indianapolis, IN: Lilly USA; May 2022.
20. Draznin B, Aroda VR, Bakris G, et al. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes–2022. Diabetes Care. 2022;45(suppl 1):S125-S143.21. GoodRx.com. Mounjaro. www.goodrx.com/mounjaro. Accessed August 1, 2022.
22. Mounjaro.com. Savings for Mounjaro. July 2022. www.mounjaro.com/savings-resources#savings. Accessed July 29, 2022.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





