US Pharm. 2015;40(9):HS22-HS24.
ABSTRACT: Human papillomavirus (HPV) infection, a prevalent issue that affects both males and females, presents in many forms (e.g., plantar warts, anogenital warts, dysplasia/neoplasms) based on location and genotype. Sexually transmitted HPV infections are typically self-limiting; however, treatment options do exist for patients experiencing bothersome symptoms. Some genotypes are responsible for the development of various cancers, most commonly cervical cancer. Current screening recommendations are intended to help with early identification of cervical cancer. Strategies aimed at reducing HPV transmission and infection include vaccination and safe sexual practices.
Human papillomavirus (HPV) is one of the most common sexually transmitted infections (STIs) in the United States. According to the CDC, most of the sexually active population are infected with HPV at some point in their lives.1 However, most individuals who are infected with HPV are asymptomatic, as <1% present with active lesions.2 There are many different genotypes of HPV (>100 types), which can manifest differently after infecting the epithelium of the skin and mucous membranes. Location also affects presentation of the infection. Common manifestations of HPV include plantar warts, anogenital warts, and dysplasia/neoplasms.3 Dysplasia, along with additional risk factors, can potentially result in cancer.2,3
HPV types 16 and 18 are associated with multiple types of cancer including cervical, anogenital (penile, vulvar, vaginal, anal), and oropharyngeal.4 Types 16 and 18 are responsible for 70% of cervical cancers, whereas types 6 and 11 are responsible for 90% of anogenital warts.3 This review will focus specifically on sexually transmitted HPV manifestations.
Most HPV infections and resulting lesions are self-limiting and resolve on their own in 1 to 2 years; therefore, treatment is often not required.2 The CDC does not recommend any specific antiviral therapy for asymptomatic or subclinical infection.4 However, there are a number of treatments available for symptomatic or bothersome lesions, none of which are curative in nature.3 Current treatment methods vary depending on manifestation and location. Selection of therapy can also be dependent on patient preference, cost, and failure of previous therapies.
Treatment of HPV Infections
External Genital Warts: There are multiple treatment options for external and perianal genital warts. Some therapies can be self-administered by patients, while other treatments require administration by healthcare providers. Per the CDC’s 2015 sexually transmitted diseases treatment guidelines, there are three recommended options for self-administration by patients: podofilox 0.5% solution or gel, imiquimod 3.75% or 5% cream, and sinecatechins 15% ointment.1 The provider-administered options include cryotherapy, trichloroacetic acid (TCA) 80%-90%, bichloroacetic acid (BCA) 80%-90%, and surgical removal.1,4 Podophyllin resin was previously included as a provider-administered option in the 2010 guidelines4; the 2015 update does not generally recommend its use because of systemic toxicities.1 TABLE 1 lists the currently recommended treatment options.1
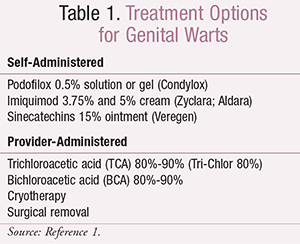
Podofilox 0.5% solution or gel (Condylox) should be applied two times daily for 3 days, followed by a period of 4 days without therapy. This dosing cycle can be repeated up to four times. The solution should be applied using a cotton swab or provided applicator, and the gel should be applied with a finger. No more than 0.5 mL of podofilox should be applied per day. Patients should be counseled that mild-to-moderate local pain or irritation may occur.1,2,4,5
Imiquimod 5% cream (Aldara) should be applied at bedtime three times per week for a maximum of 16 weeks. Imiquimod is also available as a 3.75% cream (Zyclara), which is applied every night at bedtime. Regardless of dosage strength used, patients should wash the area 6 to 10 hours after applying the medication. Patients should be counseled to expect local reactions such as redness and irritation. Hypopigmentation of the skin may also occur. It is important to educate patients that imiquimod can weaken condoms and vaginal diaphragms.1,2,4,6
Sinecatechins 15% ointment (Veregen) should be applied three times daily for a maximum of 16 weeks. Unlike imiquimod, sinecatechins ointment should not be washed off after administration. Patients should be counseled to avoid sexual contact while the ointment is on the skin. Common adverse effects include local reactions such as redness, irritation, burning, or pain. Sinecatechins is not recommended for immunocompromised patients (e.g., HIV-positive) or those with genital herpes.1,2,4,7
Provider-administered options recommended by the CDC include cryotherapy, TCA 80%-90% (Tri-Chlor 80%), BCA 80%-90%, and surgical removal.1,3,8-10 Cryotherapy involves freezing off the warts using liquid nitrogen or a cryoprobe. TCA and BCA are widely used agents to chemically remove warts. These treatments may also be used on a weekly basis as needed. Surgical removal typically only requires one office visit and is preferred in patients with many lesions. Some providers will use a combination of methods due to the failure rate of these treatments.1,4 Alternative, less common methods include intralesionally administered interferon, laser surgery, photodynamic therapy, and topical cidofovir.2,3
Internal Vaginal, Anal, and Urethral Meatus Warts: All therapies for internal vaginal and anal warts should be administered by a healthcare provider. Cryotherapy using liquid nitrogen or application of TCA or BCA 80%-90% are options for vaginal or anal warts. Additionally, surgical excision can be used for anal warts.1,3,4
As with vaginal and anal warts, treatment for urethral meatus warts is limited to provider-administered options. These options include cryotherapy with liquid nitrogen or surgical removal.1,3,4
General Patient Education and Counseling Points
Patients should be educated that HPV infections often recur regardless of treatment method utilized, especially in the first few months.2,3 When using self-applied treatment methods, patients should wash their hands before and after administration of any therapy to avoid further spreading of infection. Once diagnosed, patients with genital warts should be counseled to contact all recent sexual partners. All sexual partners can benefit from general STI screening.4 Patients with active warts should abstain from sexual activity until the warts have resolved. Surgical removal of warts can be considered in pregnant women, though efficacy is not well established. The CDC does not currently recommend podofilox or sinecatechins during pregnancy (imiquimod may be low risk). Overall, the CDC recommends avoiding drug therapy during pregnancy until more data are available.1 If precancerous lesions are detected, further follow-up is necessary to determine the appropriate course of action.
Screening for Cervical Cancer
Screening is important in detecting early cervical cancer. It is recommended that all women aged 21 to 65 years receive a Pap smear every 3 years if results are normal.1,4,11,12 Women aged ≥30 years can also elect to do DNA HPV testing in addition to the Pap smear. If they opt to have these two tests done together (i.e., cotesting), they can extend the screening interval to every 5 years if results are normal.1,4,11,12 As of 2012, screening is not recommend for women <21 years of age regardless of onset of sexual activity.11,12
According to the American Society for Colposcopy and Cervical Pathology (ASCCP) guidelines, if a woman’s Pap smear shows abnormal cytology, further evaluation may include but is not limited to colposcopy, endometrial sampling, and/or endocervical sampling.13 If a Pap smear shows unsatisfactory results (e.g., insufficient sample), a second Pap smear is recommended 2 to 4 months later, regardless of HPV status. For women aged ≥30 years who are HPV-positive and show unsatisfactory cytology, follow-up may consist of either a repeat Pap smear in 2 to 4 months or colposcopy. If the follow-up Pap smear shows further unsatisfactory results, colposcopy is recommended. For women aged ≥30 years who are HPV-positive with a normal Pap smear, repeat cotesting can be done in 1 year. If any repeat HPV test is positive or Pap smear shows significant abnormality, colposcopy is recommended.13 Depending on the severity of cervical dysplasia, treatment options may range from close monitoring for milder cases to cryotherapy, laser therapy, loop electrosurgical excision procedure (LEEP), or surgery.14
Prevention of HPV Infection and Related Diseases
Vaccination: Three HPV vaccines (Cervarix, Gardasil, and Gardasil 9) are available to help protect against anogenital infections and HPV-related diseases. A recent study showed reductions in the rates of severe cervical dysplasia cases that may be related to the introduction of HPV vaccines.15 All available vaccines are given intramuscularly (IM) as a three-dose series.16-18 The vaccines vary according to covered HPV genotypes, indicated population, and administration schedule. Differences among the vaccines are highlighted in TABLE 2. It is important to note that Cervarix is approved solely for use in females, as it protects only against the two genotypes responsible for cervical cancer. Gardasil and Gardasil 9 provide additional protection against other genotypes that are responsible for anogenital warts and related diseases (e.g., anal cancer), which allows for potential benefit in male patients.16-18
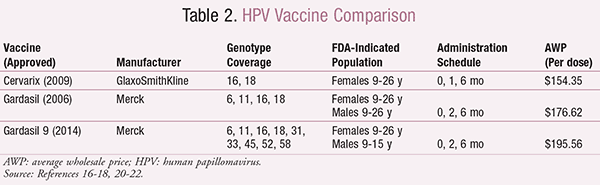
The CDC recommends routine vaccination for children (male and female) 11 to 12 years of age, in an attempt to vaccinate prior to onset of sexual activity. Catch-up vaccination is recommended for females aged 13 to 26 years, males aged 13 to 21 years, and immunocompromised males or men who have sex with men up to age 26.19 Females who have received the HPV vaccine should still be routinely screened for cervical cancer, as it can be caused by other genotypes not covered by the vaccine.3,19
The genotypes (16 and 18) covered by the HPV vaccines are responsible for the majority of cervical cancer cases. Studies have shown the vaccine efficacy rate to be above 90% in preventing infection caused by covered genotypes.3 If an individual is already infected with a specific genotype, the vaccine is not beneficial for that specific type, as it not indicated for treatment purposes. However, the vaccine can still be beneficial against other covered genotypes.3
Additional Strategies: Abstinence is an additional strategy for prevention of HPV infection. For those engaging in sexual activity, it is important to utilize safe practices to help reduce transmission of HPV or other STIs. Barrier contraception is preferred, with latex male condoms being the most effective, followed by polyurethane and female condoms. Spermicides are not recommended for reducing transmission.2
Conclusion
Anogenital warts caused by HPV infection are typically self-limiting; however, many treatment options are available for patients experiencing bothersome symptoms. None of these options is curative in nature, and oftentimes the lesions can recur. Some HPV genotypes are responsible for the development of various cancers; current screening recommendations are intended to help identify early onset of cervical dysplasia. Strategies to reduce HPV transmission and infection include vaccination, along with abstinence or safe sexual practices.
REFERENCES
1. Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(3):1-138.
2. Knodel LC. Chapter 95. Sexually transmitted diseases. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 9th ed. New York, NY: McGraw-Hill; 2014. http://accesspharmacy.mhmedical.com/content.aspx?bookid=689&Sectionid=48811491. Accessed May 4, 2015.
3. Reichman RC. Chapter 185. Human papillomavirus infections. In: Longo DL, Fauci AS, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 18th ed. New York, NY: McGraw-Hill; 2012. http://accesspharmacy.mhmedical.com/content.aspx?bookid=331&Sectionid=40726941. Accessed May 4, 2015.
4. CDC. Sexually transmitted diseases treatment guidelines, 2010. MMWR Morb Mortal Wkly Rep. 2010;59(12):1-110.
5. Podofilox. Lexi-Drugs Online. Hudson, OH: Lexi-Comp, Inc. Updated March 1, 2015. https://online.lexi.com. Accessed May 4, 2015.
6. Imiquimod. Lexi-Drugs Online. Hudson, OH: Lexi-Comp, Inc. Updated April 1, 2015. https://online.lexi.com. Accessed May 4, 2015.
7. Sinecatechins. Lexi-Drugs Online. Hudson, OH: Lexi-Comp, Inc. Updated March 19, 2015. https://online.lexi.com. Accessed May 4, 2015.
8. Podophyllum resin. Lexi-Drugs Online. Hudson, OH: Lexi-Comp, Inc. Updated May 15, 2015. https://online.lexi.com. Accessed May 18, 2015.
9. Trichloroacetic acid. Lexi-Drugs Online. Hudson, OH: Lexi-Comp, Inc. Updated April 17, 2015. https://online.lexi.com. Accessed May 18, 2015.
10. Dichloroacetic acid. Clinical Pharmacology. Tampa, FL: Elsevier. Updated September 15, 1994. www.clinicalpharmacology.com. Accessed May 18, 2015.
11. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147-172.
12. U.S. Preventive Services Task Force. Final recommendation statement: Cervical cancer: screening, March 2012. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/cervical-cancer-screening. Accessed May 4, 2015.
13. Massad LS, Einstein MH, Huh WK, et al. American Society for Colposcopy and Cervical Pathology: 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17(5 suppl 1):S1-S27.
14. Smith L. ACOG releases guidelines for managing abnormal cervical cytology and histology in adolescents. Am Fam Physician. 2006;74(8): 1431-1434.
15. Hariri S, Johnson ML, Bennett NM, et al. Population-based trends in high-grade cervical lesions in the early human papillomavirus vaccine era in the United States. Cancer. 2015 Jun 22 [Epub ahead of print].
16. Cervarix (human papillomavirus bivalent [types 16 and 18] vaccine, recombinant) package insert. Research Triangle Park, NC: GlaxoSmithKline; February 2015. www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Cervarix/pdf/CERVARIX-PI-PIL.PDF. Accessed May 4, 2015.
17. Gardasil (human papillomavirus quadrivalent [types 6, 11, 16, and 18] vaccine, recombinant) package insert. Whitehouse Station, NJ: Merck & Co., Inc; April 2015. www.merck.com/product/usa/pi_circulars/g/gardasil/gardasil_pi.pdf. Accessed May 4, 2015.
18. Gardasil 9 (human papillomavirus 9-valent vaccine, recombinant) package insert. Whitehouse Station, NJ: Merck & Co., Inc; February 2015. www.merck.com/product/usa/pi_circulars/g/gardasil_9/gardasil_9_pi.pdf. Accessed May 4, 2015.
19. Petrosky E, Bocchini JA Jr, Hariri S, et al; CDC. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2015;64(11):300-304.
20. Cervarix. Red Book Online Micromedex Solutions. Greenwood Village, CO: Truven Health Analytics. www.micromedexsolutions.com. Accessed May 4, 2015.
21. Gardasil. Red Book Online Micromedex Solutions. Greenwood Village, CO: Truven Health Analytics. www.micromedexsolutions.com. Accessed May 4, 2015.
22. Gardasil 9. Red Book Online Micromedex Solutions. Greenwood Village, CO: Truven Health Analytics. www.micromedexsolutions.com. Accessed May 4, 2015.
To comment on this article, contact rdavidson@uspharmacist.com.






