US Pharm. 2012;37(2):HS-8-HS-11.
Venous thromboembolism (VTE) occurs when a blood clot, or thrombus, occludes venous blood flow. VTE comprises deep venous thrombosis (DVT), in which the thrombus forms in the distal vein of the leg or arm, and pulmonary embolism (PE), which involves the lung. While DVT may be asymptomatic, it also can cause pain and swelling in the limb. Further, the thrombus may dislodge, travel to the lungs, and block the pulmonary vasculature, causing a potentially fatal PE. Other adverse consequences of VTE include costs associated with investigating symptomatic patients, costs and risks associated with VTE treatment, increased risk of future VTE, and the possibility of postthrombotic syndrome (characterized by chronic pain, swelling, and skin changes).1-3
Background
VTE occurs with high frequency in hospitalized patients who do not receive anticoagulant therapy. Postoperative DVT is a common complication of surgical procedures, leading to increased length of stay, excess mortality, and increased costs.1 Most hospitalized patients have at least one risk factor for VTE (TABLE 1), and the incidence of confirmed hospital-acquired DVT ranges from 10% to 40% among medical or general surgery patients and 40% to 60% among orthopedic surgery patients.1,4 The Agency for Healthcare Research and Quality identified PE—which can occur in 40% to 50% of hospitalized patients with symptomatic DVT—as the number-one cause of preventable hospital death.3,5
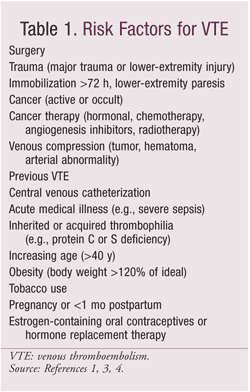
With orthopedic surgery, the majority of symptomatic VTEs occur after hospital discharge. The risk of VTE conferred by orthopedic surgery (related to continued vascular injury and impairment and to prolonged immobility) remains higher than expected for at least 2 months postoperatively, and VTE is the leading cause of hospital readmission following orthopedic surgery.1
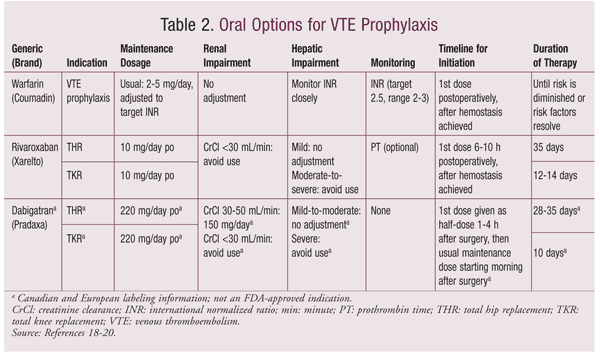
The risks of mortality, acute and long-term morbidity, and resource expenditure associated with unprevented VTE strongly support the use of thromboprophylaxis to decrease adverse outcomes, increase patient safety, and reduce costs in hospitalized patients with a moderate-to-high risk of developing VTE. Pharmacologic VTE prophylaxis lessens the risk of VTE by 50% to 60%, preventing DVT complications and morbidity and mortality from PE.1 The bleeding risk associated with pharmacologic VTE prophylaxis is low, and the cost-effectiveness of these agents has been demonstrated numerous times.1-3 Several drug classes are available, including low-dose unfractionated heparin (LDUH), low-molecular-weight heparin (LMWH), factor Xa (FXa) inhibitors, vitamin K antagonists (VKAs), and direct thrombin inhibitors (DTIs). Three of these agents—the VKA warfarin, the FXa inhibitor rivaroxaban, and the DTI dabigatran (TABLE 2)—are available in oral, rather than parenteral, dosage forms. Given that the risk of VTE extends beyond the postoperative hospital stay, the availability of these oral dosage forms may simplify extended-duration VTE prophylaxis for postsurgical patients. A discussion of the use and comparative efficacy of these agents follows.
VKAs
Safety and Efficacy Data: A randomized, double-blind trial of 1,472 patients undergoing elective total hip arthroplasty (THA) evaluated incidence of DVT and bleeding complications in patients given dose-adjusted warfarin (target international normalized ratio [INR] 2-3) postoperatively or dalteparin 2,500 U subcutaneously (SC) pre- or postoperatively and maintained on 5,000 U once daily.6 By the fifth day after surgery, 24% of warfarin patients versus 11% of preoperative and 13% of postoperative dalteparin patients (P <.001, pooled dalteparin) experienced DVT, a relative risk reduction (RRR) of between 45% and 72% favoring dalteparin. Bleeding rates were similar between groups; however, preoperative administration of dalteparin had a slightly increased risk of major bleeding compared with warfarin (P = .01).6
An open-label, randomized trial compared dose-adjusted warfarin (target INR of 2-3) administered 4 to 8 hours preoperatively and SC enoxaparin 30 mg administered twice daily in 3,011 patients undergoing elective THA.7 The primary endpoints were hospital-acquired DVT or PE and bleeding complications. At 7 days following surgery, 1% of warfarin patients versus 0.3% of enoxaparin patients experienced DVT or PE (P = .0083). Three months after discharge, the difference in VTE rates (3.7% vs. 3.6%) was no longer significant. The incidence of major bleeding episodes did not differ significantly.7
A meta-analysis of 29 trials evaluated the safety and efficacy of VKA versus other forms of thromboprophylaxis (including placebo/no treatment, dextran, antiplatelet agents, mechanical thromboprophylaxis, LDUH, and LMWH) in orthopedic surgery patients.8 Compared with placebo/no treatment, VKA yielded RRRs of 56% for DVT (P <.01) and 23% for PE (P <.01), although with a significantly higher incidence of wound hematoma. Compared with LMWH, however, VKA was associated with significantly higher rates of total and proximal DVT (9,822 patients, RR = 1.51, P <.001 and 6,131 patients, RR = 1.51, P = .028, respectively). Rates of major hemorrhage or wound hematoma did not differ significantly between VKA and LMWH.8
Summary: The VKA warfarin is significantly less effective than LMWH for preventing DVT. Rates of major bleeding are similar to those for LMWH and significantly higher than those for placebo.
FXa Inhibitors
Safety and Efficacy Data: In the Regulation of Coagulation in Orthopedic Surgery to Prevent Deep Vein Thrombosis and Pulmonary Embolism 1 (RECORD1) trial, oral rivaroxaban 10 mg once daily was compared with SC enoxaparin 40 mg once daily for the prevention of VTE in THA patients.9 Rivaroxaban was started 6 to 8 hours after wound closure. Enoxaparin was initiated 12 hours before surgery and restarted 6 to 8 hours after wound closure. The total duration of therapy was 35 days in both groups. The incidence of the primary endpoint of any DVT, nonfatal PE, or death from any cause was 3.7% in the enoxaparin group versus 1.1% in the rivaroxaban group, a significant absolute risk reduction (ARR) of 2.6% favoring rivaroxaban (P <.05).9
The RECORD2 trial aimed to determine whether 5 weeks of rivaroxaban prophylaxis was better than 2 weeks of enoxaparin prophylaxis followed by placebo in THA patients.10 The primary efficacy outcome of any DVT, nonfatal PE, or all-cause mortality (up to day 42) occurred in 2% of rivaroxaban patients and 9.3% of enoxaparin patients (P <.0001). Major VTE occurred in 0.6% of rivaroxaban patients and 5.1% of enoxaparin patients (P <.0001). Fewer rivaroxaban patients experienced symptomatic VTE.10
RECORD3 and RECORD4 evaluated rivaroxaban for use in total knee arthroplasty.11 RECORD3, a double-blind trial, randomized 2,531 patients to rivaroxaban 10 mg daily or enoxaparin 40 mg daily for 10 to 14 days. The composite primary efficacy outcome of any DVT, nonfatal PE, or death from any cause (within 13-17 days after surgery) occurred in 9.6% of rivaroxaban patients and 18.9% of enoxaparin patients (P <.001), a 62% RRR in major VTE in favor of rivaroxaban. RECORD4 compared rivaroxaban to enoxaparin 30 mg twice daily for 10 to 14 days.12 Rivaroxaban was superior to twice-daily enoxaparin for preventing DVT, nonfatal PE, or death from any cause up to day 17 after surgery (incidence of primary composite outcome was 6.9% vs. 10.1%, respectively). There were no statistically significant differences between rivaroxaban and enoxaparin in the incidence of major VTE, symptomatic VTE, or major bleeding in any of the RECORD studies.
Summary: The oral FXa inhibitor rivaroxaban is statistically superior to enoxaparin for preventing VTE in hip replacement and knee replacement patients without increasing the risk of bleeding.
Direct Thrombin Inhibitors
Safety and Efficacy Data: The RE-NOVATE study compared the efficacy and safety of two dosages of dabigatran etexilate (220 mg and 150 mg) versus enoxaparin 40 mg once daily for 1 month to reduce VTE risk after hip replacement surgery.13 This randomized, double-blind, noninferiority study demonstrated dabigatran’s noninferiority in the primary efficacy outcome of total VTE and all-cause mortality during treatment, with no difference in major VTE or VTE-related death. Results favored dabigatran 220 mg over 150 mg.13
RE-NOVATE II compared dabigatran 220 mg daily with enoxaparin 40 mg daily for 1 month after primary total hip replacement (THR).14 This study showed noninferiority of dabigatran 220 mg to enoxaparin for the primary endpoint of total VTE and all-cause mortality. Dabigatran also demonstrated a statistically significant 1.1% ARR in the prevention of major VTE and VTE-related mortality.14
RE-MOBILIZE, a randomized, double-blind, active-controlled, noninferiority study, evaluated dabigatran 220 mg once daily, dabigatran 150 mg once daily, and enoxaparin 30 mg twice daily for VTE prevention after knee arthroplasty.15 Dabigatran was administered 6 to 8 hours after surgery at half the study dose, with full dosing beginning the morning following surgery. The first enoxaparin dose was given the morning following surgery, and treatment was continued for 12 to 15 days. The primary efficacy outcome of total VTE and all-cause mortality during treatment occurred in 31.1% of patients taking dabigatran 220 mg, 33.7% of those taking 150 mg, and 25.3% of those taking enoxaparin. Both dabigatran dosages failed to demonstrate noninferiority to enoxaparin 30 mg twice daily. The secondary endpoint of major VTE and VTE-related mortality occurred in 3.4% and 3.0% of patients taking dabigatran 220 mg and 150 mg, respectively, versus 2.2% of the enoxaparin group.
There was no statistically significant difference in the safety endpoint of major bleeding in any trial comparing dabigatran with enoxaparin.13-15
Summary: Dabigatran, an oral DTI, has a safety profile similar to that of enoxaparin for VTE prophylaxis following both hip replacement and knee replacement. Dabigatran has proven noninferiority to enoxaparin following hip replacement surgery, but at similar dosages has failed to show comparable efficacy following knee replacement surgery.
Duration of Antithrombotic Therapy
In one epidemiologic study, 76% of VTEs were diagnosed after discharge in almost 20,000 patients who underwent hip replacement surgery.16 The rate of post–hospital discharge VTE in the approximately 24,000 knee surgery patients enrolled in the study was slightly less than the rate in THR patients (2.1 vs. 2.8%, P <.05). VKAs given after hospital discharge have demonstrated successful reductions in postdischarge VTE incidence in orthopedic surgery patients. In a trial of VKA prophylaxis (target INR 2-3) discontinued at discharge or continued for an additional 4 weeks postdischarge, patients receiving extended-duration warfarin experienced significantly fewer VTEs (0.5% vs. 5.1%, P <.05).17 Several other trials, literature reviews, and meta-analyses—including recent trials of rivaroxaban—have found that postdischarge thromboprophylaxis is safe and effective for reducing VTE incidence in patients recently discharged from the hospital.10 Current guidelines for the prevention of VTE recommend prophylaxis of at least 10 days for all orthopedic surgeries and advise extended-duration prophylaxis (up to 35 days) for patients undergoing THR or hip fracture surgery.1-3
Conclusion
The risk of VTE following orthopedic surgery is high, but pharmacologic prophylaxis significantly reduces the incidence of VTE and decreases the associated morbidity, mortality, and costs without putting patients at excessive risk for bleeding. Recent data suggest that some patients, particularly those who are undergoing THR, may benefit from extended-duration prophylaxis. The availability of anticoagulants with oral dosage forms could simplify medication regimens for patients receiving VTE prophylaxis after hospital discharge. While warfarin is available for use in such situations, it is less effective than LMWH for preventing VTE; it also requires frequent and complex monitoring.
Rivaroxaban is more effective than LMWH for VTE prophylaxis. According to phase III trials, rivaroxaban does not increase the risk of major bleeding compared with other agents, and it has been studied in extended-duration prophylaxis. Dabigatran has efficacy equivalent to that of LMWH in patients undergoing hip replacement, but not in those undergoing knee replacement surgery. Currently, warfarin and rivaroxaban are the only two FDA-approved oral agents for postoperative VTE prophylaxis; warfarin demonstrates statistical inferiority to LMWH, while rivaroxaban appears superior to LMWH for this indication. Future studies should evaluate the cost-effectiveness of using oral agents, particularly rivaroxaban and dabigatran, for extended-duration prophylaxis of VTE following orthopedic surgeries.
REFERENCES
1. Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:381S-453S.
2. National Institute for Health and Clinical Excellence. Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital.
http://guidance.nice.org.uk/
3. Maynard G, Stein J. Preventing Hospital-Acquired Venous Thromboembolism: A Guide for Effective Quality Improvement. AHRQ Publication No. 08-0075. Rockville, MD: Agency for Healthcare Research and Quality; August 2008.
4. Anderson FA Jr, Wheeler HB, Goldberg RJ, et al. The prevalence of risk factors for venous thromboembolism among hospital patients. Arch Intern Med. 1992;152:1660-1664.
5. Kearon C. Natural history of venous thromboembolism. Circulation. 2003;107:122-130.
6. Hull RD, Pineo GF, Francis C, et al. Low-molecular-weight heparin prophylaxis using dalteparin in close proximity to surgery vs warfarin in hip arthroplasty patients: a double-blind, randomized comparison. Arch Intern Med. 2000;160:2199-2207.
7. Colwell CW Jr, Collis DK, Paulson R, et al. Comparison of enoxaparin and warfarin for the prevention of venous thromboembolic disease after total hip arthroplasty. Evaluation during hospitalization and three months after discharge. J Bone Joint Surg Am. 1999;81:932-940.
8. Mismetti P, Laporte S, Zufferey P, et. al. Prevention of venous thromboembolism in orthopedic surgery with vitamin K antagonists: a meta-analysis. J Thromb Haemost. 2004;2:1058-1070.
9. Eriksson BI, Borris LC, Friedman RJ, et al; RECORD1 Study Group. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358:2765-2775.
10. Kakkar AK, Brenner B, Dahl OE, et al; RECORD2 Investigators. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet. 2008;372:31-39.
11. Lassen MR, Ageno W, Borris LC, et al; RECORD3 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med. 2008;358:2776-2786.
12. Turpie AG, Lassen MR, Davidson BL, et al; RECORD4 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet. 2009;373:1673-1680.
13. Eriksson BI, Dahl OE, Rosencher N, et al; RE-NOVATE Study Group. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet. 2007;370:949-956.
14. Eriksson BI, Dahl OE, Huo MH, et al; RE-NOVATE II Study Group. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*). A randomised, double-blind, non-inferiority trial. Thromb Haemost. 2011;105:721-729.
15. Friedman RJ, Dahl OE, Rosencher N, et al; RE-MOBILIZE, RE-MODEL, RE-NOVATE Steering Committees. Dabigatran versus enoxaparin for prevention of venous thromboembolism after hip or knee arthroplasty: a pooled analysis of three trials. Thromb Res. 2010;126:175-182.
16. White RH, Romano PS, Zhou H, et al. Incidence and time course of thromboembolic outcomes following total hip or knee arthroplasty. Arch Intern Med. 1998;158:1525-1531.
17. Prandoni P, Bruchi O, Sabbion P, et al. Prolonged thromboprophylaxis with oral anticoagulants after total hip arthroplasty: a prospective controlled randomized study. Arch Intern Med. 2002;162:1966-1971.
18. Coumadin (warfarin) package insert. Princeton, NJ: Bristol-Myers Squibb; October 2011.
19. Xarelto (rivaroxaban) package insert. Titusville, NJ: Janssen Pharmaceuticals, Inc; November 2011.
20. Pradaxa (dabigatran) package insert. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; November 2011.
To comment on this article, contact rdavidson@uspharmacist.com.





