US Pharm. 2022;47(4):27-32.
Human papillomavirus (HPV) is the most prevalent sexually transmitted infection (STI) worldwide.1-3 In the United States, 79 million people are infected with HPV, with 50% of infections occurring prior to age 24 years.2,4 It is estimated that up to 80% of sexually active people will become infected with HPV during their lifetime.5 Annually, 14 million new infections occur in sexually active people in the U.S.5,6
The prevalence of vaginal HPV in women is 26.8%, while men experience a high burden of HPV penile infection at 45.2%.2 A recent study revealed that 50% of women infected with HPV are middle-aged adults ranging from age 27 to 45 years.7 Prevalence of HPV in men is bimodal, peaking at ages 28 to 32 years (50.8%) and again at ages 58 to 59 years (59.6%).2 The prevalence of HPV in transgender people is estimated to be between 40% and 95%.9 Men and women aged 50 to 54 years have the highest prevalence of high-risk (HR) HPV infection of the oropharynx.7 Unlike other STIs, the HPV infection rate remains high across all ages.4
HPV is a member of the papillomavirus family and only infects humans. Transmission occurs during vaginal or anal intercourse, but HPV can also be transmitted via oral-genital or genital-genital contact as a product of mucosal trauma.6,8 Thus, the HPV transmission rate reported by women who have only had sex with women is no different compared with heterosexual women.3 HPV can infect basal cells of the vagina, vulva, cervix, neocervix, intestinal vaginoplasty, anus, oropharynx, penis, and perianal region.6,9 Initially, HPV invades the basal cells of mucosal epithelium, integrating viral DNA into the host genome. The virus replicates slowly, maintaining a low copy number and resulting in minimal viral protein generation, which evades host humoral immune detection. Most HPV infections are transient, lasting 8 to 13 months. Once detected, the host’s cell-mediated immunity resolves the infection within 5 to 8 months, or the virus becomes dormant.6 Men mount a weaker immune response when infected with HPV, developing lower circulating HPV antibodies and acting as a reservoir for HPV.2 Immunocompromised individuals are unable to clear HPV infections quickly and may be at greater risk for developing sequelae.2,6,10
There are more than 200 HPV serotypes, of which at least 40 are transmitted through direct sexual contact.10 These 40 serotypes are categorized into two groups based on the risk of developing cancer following infection. Low-risk HPV serotypes include 6 and 11, which cause 90% of anogenital warts, low-grade changes in cervical cells, and recurrent respiratory papillomatosis.2,3,6 Other low-risk serotypes (i.e., 1, 2, 3, 4, 27, and 57) affect the cutaneous squamous epithelium of skin, leading to warts (e.g., plantar digital, flat).6 High-risk HPV serotypes include 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 68, 73, and 82. These serotypes are also known as the oncogenic HPV serotypes and are implicated in vaginal, cervical, vulvar, anal, penile, and oropharyngeal cancers.4,10 Development of cancer is slow on average, as women test positive for an oncogenic HPV serotype 3 to 5 years prior to the incident cancer.4 The median age for HPV-associated oropharyngeal cancers is 61 years in men and 63 years in women, occurring well after initial infection to HPV.10
Risk factors for infection with HPV are strongly associated with sexual behaviors and sexual history.8 Inconsistent condom use, smoking, sex with a new partner, and lifetime number of partners are important risk factors for women and men.7,8 Men who are single, never married, living with a partner, or recently separated are twice as likely to be infected with genital HPV.2,7 Observational studies have reported detection of HPV in 45% of females prior to their first vaginal sexual experience.3 HPV inflection can resolve and be cleared, persist at an undetectable level, or become latent.11 Latent infections are nondetectable until the host becomes immune compromised. HPV can be detected in up to 20% of women who have cleared the infection.3,11 HPV status is difficult to measure, as cervical screening tests are not able to differentiate between a new infection, reinfection, or reactivated infection, and screening recommendations are lacking for other HPV-related cancers.8 In addition, screening methods may need to be adjusted for the impact that 15 years of widespread vaccination will have on herd immunity and the natural progression of HPV.8,11
Impact and Consequences of HPV
HPV infections usually resolve within 2 years, with only 1% progressing to invasive cancer.6 HPV is related to 90% of cervical and anal cancers, 70% of oropharyngeal, vaginal, and vulvar cancers, and 60% of penile cancers.10 Over 50% of cancers related to HPV are caused by serotypes 16 and 18.4 The most recent data from the U.S. Cancer Statistics Data Brief report an incidence of 46,143 cases of HPV-associated cancers annually. Women account for 25,719 of total cases each year, with cervical cancer representing 48% (12,200 cases annually) of female HPV-related cancers.10 Men are reported to represent 20,424 of HPV-related cancer cases annually, with 82% (16,680 cases each year) being oropharyngeal cancers.10 The American Cancer Society estimates there will be an increase to 54,000 new cases of HPV-related oropharyngeal cancers in 2022.12
It is also important to note that certain populations are presenting with more HPV-related cancers than reported in the past.3 For example, the anal cancer rate ratios of HIV-positive men and HIV-positive men having sex with men are 26.7 and 80.3, respectively, compared with HIV-negative men.3 Women taking antiretroviral therapy for HIV experience a higher incidence of anogenital warts (28.4% compared with 9.3% of HIV-negative women).3 Transgender women and men experience a high HPV exposure rate and have limited access to and use of screening methods.9 Among transgender women, the presence of multiple HPV serotypes was found in 40% to 66% of the population, including the major oncogenic strains HPV 16 and HPV 18.9,10
HPV Prevention
Preventative measures for HPV infection include safe- sex education, screening, and vaccination against specific serotypes of HPV.13,14 Encouraging consistent condom use and male circumcision can reduce the risk of transmission of HPV.13 Routine screening for HPV is only conducted for detection of cervical cancer.13,14 Since 1976, organized screening programs using the Papanicolaou (“pap”) smear in the U.S. are believed to have prevented well over 500,000 cases of cervical cancer and to have reduced cervical cancer rates related to racial disparities.14
Cytologic testing has a high specificity (96%) but a low sensitivity ranging from 19% to 77% for the detection of cervical intraepithelial neoplasia 2 (CIN2) or greater. Low sensitivity is overcome by more frequent testing (e.g., every 2-3 years).13 Testing for HPV DNA every 5 years is now recommended for primary screening in women aged 30 years or older in the U.S. due to increased sensitivity for detection of high-grade cervical lesions, which are more prevalent in this age group.13,14 Interestingly, DNA tests lack specificity, which leads to more false-positive results. Therefore, cytologic testing is recommended simultaneously.13 Careful application of screening guidelines decreases the possibility of detecting and treating lesions that would resolve with time while detecting high-grade cervical lesions that lead to invasive cancers.13,14
National guidelines or recommendations regarding routine screening for anal, oropharyngeal, or penile HR-HPV are lacking. However, the American College of Obstetrics and Gynecology committee on Health Care for Underserved Women recommends cervical screening for transgender men with cervices and pap testing for transgender women with neocervices.9 Additional research into the natural progression of HR-HPV to cancer is needed to develop sensitive and specific screening tests and to efficiently deploy them.3
Vaccines
Since 2006, primary prevention for cervical cancer has shifted from screening to vaccination. Prior to the availability of HPV vaccines, the prevalence of HPV 6, 11, 16, and 18 was 11.5% and 18.5% in females aged 14 to 19 years and 20 to 24 years, respectively.4 As of 2016, the prevalence in females decreased to 1.8% in females aged 14 to 19 years and 5.3% in females aged 20 to 24 years.4 Epidemiological studies project that a vaccination rate of 40% reduces the relative risk of HPV 16 infection to 0.18% in vaccinated and 0.35% in unvaccinated individuals.4
The quadrivalent Gardasil (Merck & Co., Inc.) vaccine became available in 2006 for females aged 9 to 25 years.15 This first-of-its-kind cancer-preventing vaccine targeted the four most pathogenic HPV serotypes 6, 11, 16, and 18.4,15 A double-blind, placebo-controlled multinational study of 12,000 women aged 15 to 26 years receiving the quadrivalent vaccine reported 98% efficacy in the prevention of the combined outcome for CIN2, CIN3, and adenocarcinoma in-situ (AIS) in women aged 15 to 26 years with no history of abnormal pap smear result and fewer than four sexual partners.14 In the same study, the vaccine was found to be 42% and 79% effective in prevention of HPV-16 and HPV-18, respectively.14 The vaccine prevention rates against CIN2/3 were 57% for HPV-16 and 45% for HPV-18 associated lesions.4
In 2009, the bivalent Cervarix (GlaxoSmithKline Pharmaceuticals, Ltd.) vaccine became available for females aged 9 to 26 years.16 The formulation provided protection against serotypes 16 and 18, which are responsible for most cases of HPV-associated cancer.17 Efficacy of the bivalent vaccine was studied in 18,000 females aged 9 to 26 years in a double-blind, placebo, multinational study.18 The bivalent vaccine was 92.9% effective in preventing CIN2 in women 15 to 25 years with normal- to low-grade cervical dysplasia and 52.8% effective in preventing CIN2 in females with a history of HPV.18 In a 9-year follow-up of HPV-naïve patients, vaccine efficacy was 100% against HPV 16 and 18.18 However, there was no difference in the CIN2 or CIN3 rates. The lack of difference was attributed to a low overall rate of CIN2 and CIN3.4,18
In 2014, Gardasil 9 (Merck & Co., Inc.) was approved by the FDA for the prevention of cervical, vulvar, vaginal, and anal cancers caused by HPV 16, 18, 31, 33, 45, 52, and 58, as well as the prevention of genital warts caused by HPV 6 or 11, in females aged 9 to 26 years and males aged 9 to 15 years.19,20 This vaccine is nonvalent (9vHPV vaccine) with protection against five serotypes of HPV (31, 33, 45, 52, and 58) that cause approximately 20% of cervical cancers and were not covered by the previously approved HPV vaccines.19 In a randomized, double-blind, noninferiority trial comparing 9vHPV vaccine with the quadrivalent (4vHPV) vaccine in women aged 16 to 26 years, the 9vHPV vaccine prevented 96% of high-grade CIN, AIS, vulvar, and vaginal cancers associated with targeted HPV serotypes. The 9vHPV vaccine also reduced the rate of high-grade CIN, AIS, vulvar, and vaginal cancers from 1.5 to 0.1 cases of per 1,000 person-years and was found to be 96% effective against persistent infections, reducing the infection rate from 52.4 to 2.1 cases per 1,000 person-years. In patients with prior HPV infection, efficacy was no different between the 4vHPV and 9vHPV vaccines.4,18
All HPV vaccines provide 90% to 100% efficacy against their respective HPV serotypes when administered to HPV-naïve patients.18 Efficacy has been found to decrease to 58% if patients are infected with HPV 16 and/or 18 prior to vaccination.4 HPV vaccination in men was found to be 85.6% effective in preventing persistent HPV, 78% effective in preventing anogenital cancer, and 82.4% effective against oropharyngeal cancers caused by HPV 16 and/or 18.18,21
HPV vaccine efficacy has been thoroughly studied in cisgender, heterosexual women. However, efficacy outcomes in men, men having sex with men (MSM), HIV-positive men, and transgender individuals are lacking.3,7,9,22 Thus, more research studies are needed for HPV vaccine recommendations in these populations.23
Vaccination in men has been determined to be moderately effective against HPV genital infection and anal intraepithelial lesions (28%-49.5%), irrespective of HPV status.23 Vaccine effectiveness against anogenital cancer and dysplasia in HPV-naïve men is considerably higher at 78%.4,23 A 79% efficacy rate against genital lesions and a decrease in risk of recurrent high-grade anal intraepithelial neoplasia have been seen in MSM.18 However, despite a seroconversion rate of 96% to 100% following 9vHPV vaccination, the durability of the antibody response is shortened by the lack of herd immunity.18,23 Thus, MSM may need extended access to vaccination through the fifth decade of life.18 Overall, data on the efficacy of HPV vaccination in transgender people are lacking.
Currently, the 9vHPV vaccine (Gardasil 9, Merck & Co., Inc.) is the only vaccine available in the U.S.19,24 Recommendations for which patients should receive HPV vaccine for prophylaxis have evolved with the recognition of risk factors and a greater understanding of HPV infection (see TABLE 1). The Advisory Committee on Immunization Practices (ACIP) recommends routine vaccination at age 11 or 12 years, with the option to initiate the vaccination series at age 9 years.25 Catch-up vaccination is available and recommended for all through age 26 years.26 Persons outside of this age range (27-45 years) and those inadequately vaccinated may be at risk for new HPV infection.25,26 The ACIP recommends shared clinical decision making with the primary provider to determine the benefit from vaccination.26 Health economic analysis using current ACIP recommendations for vaccination calculated the number needed to vaccinate (NNV) to prevent one case of anogenital warts, CIN2 or worse, or cancer as 9, 22, and 202, respectively. If recommendations were expanded to include all adults age 26 through 45 years, the NNV changes to 120, 800, and 6,500 respectively.1,26
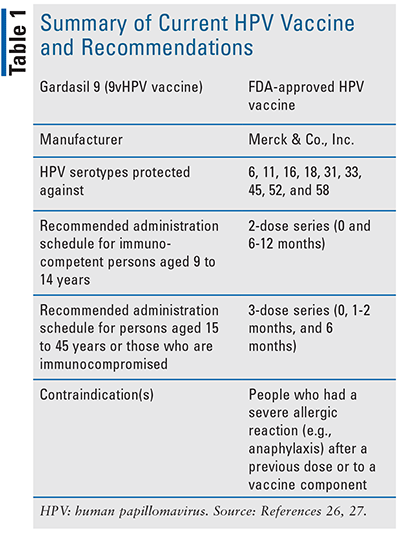
Vaccination Schedules
Vaccination schedules differ by age of initiation of the series and certain risk factors.1,26 For children and adolescents aged 9 to 14 years, two doses of the 9vHPV vaccine are recommended at least 6 months apart. Adolescents and adults aged 15 to 45 years require three doses (0, 1-2, and 6 months apart) to achieve the same level of protection as in younger adolescents.26,27 Persons with primary or secondary immunocompromising conditions who might have reduced cell-mediated or humoral immunity where immune response to vaccination may be attenuated (e.g., B lymphocyte antibody deficiencies, T lymphocyte complete or partial defects, HIV infection, malignant neoplasm, transplantation, autoimmune disease, or immunosuppressive therapy) should also receive the three-dose series.27 The duration of vaccine effectiveness remains above 90%, with no waning of immunity through at least 10 to 12 years after immunization.1
Vaccination Campaigns
Vaccination campaigns have successfully motivated the adolescent and young adult populations to get vaccinated.1,28 There has been a focus on the mainstream community failing to address and inform certain populations (e.g., minority groups) about HPV and vaccination.28 Prior to the implementation of such campaigns, only 25% of adolescents aged 13 to 18 years and adults aged up to 20 years had ever heard about HPV and prophylactic vaccination.28 One focus group of MSM median aged 25 years revealed that 75% did not know HPV could infect men, could not estimate their risk for infection, or did not know HPV is the cause of genital warts.22 Over 90% did not know HPV could cause anal cancer or that MSM are 17 times more likely to develop anal cancer.22 Despite 79% knowing nothing about the HPV vaccine, most wanted to get the vaccine once they learned it was available.22 The transgender community faces similar challenges related to awareness of HPV, availability of a vaccine, and access to healthcare providers trained in the care of transgender patients.29 After implementation, 71.5% of adolescents (aged 13-17 years) were reported to have received at least one dose of HPV vaccine, and 54.2% had completed the series.1 Among the young adult population (aged 18-26 years), 56.8% of females and 51.8% of males had completed the series.1
Role of the Pharmacist
In the President’s Cancer Panel Report of 2013, pharmacies were designated as points of access available to most people in the U.S.7,17 Pharmacists are competent immunizers and, in certain geographical areas, the most accessible healthcare providers.29,30 They may be available on weekdays, weekends, and holidays and have extended hours for their patients. In addition, appointments for vaccinations are not always required.29,30 During the COVID-19 pandemic, community pharmacists have administered more than 227 million doses of vaccines against COVID-19.29 Forty states have laws authorizing pharmacists to administer HPV vaccinations. However, only 22 of those allow pharmacists to administer the vaccine to patients aged 11 or 12 years.30 The remaining 18 states authorize pharmacists to vaccinate older adolescents, creating a barrier to vaccination prior to sexual debut as recommended by the CDC.30 Expanding pharmacists’ vaccination authority to preadolescents provides improved access for susceptible patients and reduces healthcare costs related to provider office visits.30 Other obstacles identified can be overcome with consistent education and messaging.29,30 Changing the narrative to focus on prevention of cancer may take the stigma away from getting vaccinated.4,22,28,29
Conclusion
The vast majority of sexually active people will become infected with HPV during their lifetime.4 As one of the most accessible healthcare professionals, pharmacists should be prepared to educate the public on HPV and its prevention. Pharmacists should also recommend and administer HPV vaccinations, when appropriate. In addition, all healthcare professionals should encourage open and honest dialogue with their patients to minimize the stigma that exists around HPV and other STIs.
REFERENCES
1. Meites E, Gee J, Unger E, Markowitz L. Human papillomavirus. In: Epidemiology and prevention of vaccine-preventable diseases (The Pink Book). www.cdc.gov/vaccines/pubs/pinkbook/hpv.html. Accessed February 10, 2022.
2. Han JJ, Tarney CM, Song J. Variation in genital human papillomavirus infection prevalence and vaccination coverage among men and women in the USA. Future Oncol. 2017;13(13):1129-1132.
3. Dunne EF, Park IU. HPV and HPV-associated diseases. Infect Dis Clin North Am. 2013;27(4):765-778.
4. St. Laurent J, Luckett R, Feldman S. HPV vaccination and the effects on rates of HPV-related cancers. Curr Probl Cancer. 2018;42(5):493-506.
5. Cleveland Clinic. HPV (human papilloma virus). www.my.clevelandclinic.org/health/diseases/11901-hpv-human-papilloma-virus. Accessed February 10, 2022.
6. Brianti P, De Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol. 2017;40(2):80-85.
7. Prabhu VS, Roberts CS, Kothari S, Niccolai L. Median age at HPV infection among women in the United States: a model-based analysis informed by real-world data. Open Forum Infect Dis. 2021;8(7):1-8.
8. Chandler E, Ding L, Gorbach P, et al. Epidemiology of any and vaccine-type anogenital human papillomavirus among 13 to 26-year-old young men after HPV vaccine introduction. J Adolesc Health. 2018;63(1):43-49.
9. Brown B, Poteat T, Marg L, et al. Human papillomavirus-related cancer surveillance, prevention, and screening among transgender men and women: neglected populations at high risk. LGBT Health. 2017;4(5):315-319.
10. CDC. Cancers associated with human papillomavirus, United States 2013-2017. www.cdc.gov/cancer/uscs/about/data-briefs/no18-hpv-assoc-cancers-UnitedStates-2013-2017.htm. Accessed February 10, 2022.
11. Gravitt PE, Winer RL. Natural history of HPV infection across the lifespan: role of viral latency. Viruses. 2017;9(10):267.
12. American Cancer Society. Key statistics for oral cavity and oropharyngeal cancers. www.cancer.org/cancer/oral-cavity-and-oropharyngeal-cancer/about/key-statistics.html. Accessed February 10, 2022.
13. Dahlstrom KR, Day AT, Sturgis EM. Prevention and screening of HPV malignancies. Semin Radiat Oncol. 2021;31:297-308.
14. Yang DX, Soulos PR, Davis B, et al. Impact of widespread cervical cancer screening numbers of cancers prevented and changes in race-specific incidence. Am J Clin Oncol. 2018;41(3):289-294.
15. FDA. Gardasil. www.fda.gov/vaccines-blood-biologics/vaccines/gardasil. Accessed February 10, 2022.
16. FDA. Cervarix. www.fda.gov/vaccines-blood-biologics/vaccines/cervarix. Accessed February 10, 2022.
17. National Cancer Institute. HPV and cancer. www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-and-cancer. Accessed February 10, 2022.
18. Kamolratanakul S, Pitisuttithum P. Human papillomavirus vaccine efficacy and effectiveness against cancer. Vaccines (Basel). 2021;9(12):1413.
19. FDA. Gardasil 9. www.fda.gov/vaccines-blood-biologics/vaccines/gardasil-9. Accessed on February 10, 2022.
20. Merck & Co., Inc. Gardasil 9. www.merckvaccines.com/gardasil9/. Accessed February 10, 2022.
21. Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices. Morb Mortal Wkly Rep. 2014;63:1-30.
22. Nadarzynski T, Smith H, Richardson D, et al. Perceptions of HPV and attitudes towards HPV vaccination amongst men who have sex with men: a qualitative analysis. Br J Health Psychol. 2017;22(2):345-361.
23. Harder T, Wichmann O, Klug SJ, et al. Efficacy, effectiveness and safety of vaccination against human papillomavirus in males: a systematic review. BMC Med. 2018;16(1):110.
24. CDC. HPV vaccination recommendations. www.cdc.gov/vaccines/vpd/hpv/hcp/recommendations.html. Accessed February 10, 2022.
25. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. Morb Mortal Wkly Rep. 2019;68:698-702.
26. CDC. Administering HPV vaccine. www.cdc.gov/vaccines/vpd/hpv/hcp/administration.html. Accessed February 10, 2022.
27. Thanasas I, Lavranos G, Gkogkou P, et al. Understanding of young adolescents about HPV infection: how health education can improve vaccination rate. J Cancer Educ. 2020;35(5):850-859.
28. Weyers S, Garland SM, Cruickshank M, et al. Cervical cancer prevention in transgender men: a review. BJOG. 2021;128(5):822-826.
29. CDC. Epidemiology and prevention of vaccine-preventable diseases. Hall E, Wodi AP, Hamborsky J, et al, eds. 14th ed. Washington, DC Public Health Foundation, 2021.
30. Dingman D, Schmit CD. Authority of pharmacists to administer human papillomavirus vaccine: alignment of state laws with age-level recommendations. Public Health Rep. 2-18;133:55-63.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





