US Pharm. 2021;46(11):HS7-HS12.
ABSTRACT: Hyperglycemia is a leading cause of increased morbidity and mortality in critically ill diabetic and nondiabetic patients in the ICU. Stricter control should be implemented in this setting in order to reduce mortality as well as other complications caused by hyperglycemia. Because hypoglycemia is associated with an increased risk of adverse effects, the optimal intensity of glucose control has been extensively investigated. Hyperglycemia is better controlled through continuous glucose infusions than with intermittent injections or IV infusions because it is easier to titrate the concentration of insulin to achieve a target glucose range. Pharmacists in acute-care and ambulatory-care settings are able to adjust insulin therapy and educate patients about hypoglycemia or hyperglycemia in order to optimize patient outcomes.
Hyperglycemia induced by critical illnesses (trauma, stroke, myocardial infarction, and sepsis) was previously considered a benign survival response to the disruption of homeostasis.1 The normoglycemic range for critically ill patients has been a controversial topic and has sparked multiple studies to determine an optimal range for those in the ICU.2 Depending on the type of ICU (e.g., medical ICU [MICU], surgical ICU [SICU]) and whether the patient is diabetic or nondiabetic, the glycemic ranges and hyperglycemia management could differ slightly, but these patients are often treated with exogenous insulin.2
Importance of Tight Glucose Control
The use of tight glucose control contributes to decreased morbidity and mortality in hospitalized patients.3 Hyperglycemia leads to various complications, such as increased risk of infections, kidney damage, adrenal failure, and organ failure. In a 2006 study of 767 critically ill patients who were in the ICU for more than 3 days, there was a 10% reduction in morbidity and mortality in the 386 patients who underwent intensive glucose control. Reductions in morbidity included lower rates of kidney injury or failure, quicker decannulation from ventilators, and early discharge from the ICU to the floor.4
Epidemiology
The prevalence of hyperglycemia in critically ill patients is difficult to ascertain because of the different thresholds used to determine abnormal glucose concentrations. However, trauma patients in the emergency department tend to have much higher mortality rates because of poorer glucose control due to lack of management.5 Patients (whether diabetic or not) may have a blood glucose concentration ranging from 120 mg/dL to 200 mg/dL upon hospital admission. In one study, 74.5% of patients had a blood glucose concentration >110 mg/dL and 12% had a concentration >200 mg/dL upon admission to the SICU.6 Another study found that the average blood glucose concentration upon admission to the MICU was 162 mg/dL.4 In SICU and MICU patients, the average blood sugar concentration was 145 mg/dL.7 In these studies, cardiac surgical patients were also included in analyses.
Etiology and Pathophysiology
The etiology of hyperglycemia in critically ill patients has been shown to be multifactorial. Whether diabetic or nondiabetic, patients can develop stress-induced hyperglycemia because of the body’s response to the disruption in homeostasis. This may occur as an endocrine, metabolic, and/or immune response to a critical illness. Stress responses initially activate the hypothalamus and the release of catecholamines, such as epinephrine. This causes the respiratory rate and the heart rate to increase; however, prolonged stress can also activate the hypothalamic-pituitary-adrenal axis, prompting the release of cortisol.8 Cortisol’s role in stress triggers gluconeogenesis and glycogenolysis, providing enough energy for the body to use while under stress. The release of counterregulatory hormones (glucagon, catecholamines, and growth hormone) may stimulate lipolysis of adipose tissue.8
There is evidence of increased expression of tumor necrosis factor-alpha (TNF-alpha), interleukin (IL)-1, and IL-6 in adipose tissue.9 This dysregulation of adipose-tissue lipolysis results in elevated concentrations of fatty acids in the circulatory system. Lipids may accumulate in insulin-sensitive organs such as the liver and skeletal muscle, causing insulin resistance.10 Other causes of insulin resistance include bed rest, impaired insulin-receptor binding, and disruption of the insulin-signaling pathway.1 Epinephrine can inhibit insulin-stimulated glucose transport in the skeletal muscle, whereas TNF-alpha and IL-1 cytokines can inhibit postreceptor insulin signaling.1 Medications given in the hospital setting to treat a critical illness (exogenous glucocorticoids, vasopressors, lithium, and beta-blockers) can lead to stress-induced hyperglycemia.1 These factors may enhance the effects of the ongoing stress response.
Evidence for Glucose Control
The 2001 Leuven Surgical Trial was a single-center, nonblinded, randomized, controlled study that assessed ICU mortality between different serum glucose ranges in the SICU in 1,548 patients undergoing intensive versus lenient glucose control.6 This study showed that intensive insulin therapy (IIT; serum glucose goal 80 mg/dL-110 mg/dL) reduced mortality compared with conventional therapy (serum glucose goal 180 mg/dL-200 mg/dL) in patients who spent more than 5 days in the SICU (4.6% with IIT and 8% with conventional therapy, P <.04).6
The 2006 Leuven Medical Trial was a single-center, nonblinded, randomized, controlled study of 1,200 patients that assessed death from any cause in the hospital between different serum glucose ranges in the MICU. This study found that IIT (serum glucose goal 80-110 mg/dL) did not reduce mortality compared with conventional therapy (serum glucose goal 180-200 mg/dL) in patients who stayed in the MICU <3 days (40.0% in the conventional-treatment group vs. 37.3% in the IIT group, P = .33).4 Although length of hospital stay can be difficult to predict, this study showed that in-hospital mortality and morbidity were reduced in patients on IIT who spent 3 or more days in the ICU (43.0% for IIT and 52.5% for conventional therapy, P = .009).
The merits of implementing intensive glucose control will depend on the situation, as indicated in the studies discussed above. The 2008 VISEP (Efficacy of Volume Substitution and Insulin Therapy in Severe Sepsis) study measured the safety of IIT versus conventional glucose management in 537 ICU patients.11 This multicenter, randomized, controlled, two-by-two factorial trial was stopped early because of the increased rates of hypoglycemia (17.0% for IIT vs. 4.1% for conventional treatment, P <.001) and serious adverse events (10.9% for IIT vs. 5.2% for conventional treatment, P = .01) as well as lack of a survival benefit.11 These results were utilized to revise the 2016 Surviving Sepsis Campaign guidelines, in which the recommendation for glucose control was changed from targeting serum glucose concentrations of 110 mg/dL or lower to concentrations of 180 mg/dL or lower.12
NICE-SUGAR (Normoglycemia in Intensive Care Evaluation–Survival Using Glucose Algorithm Regulation) was a multicenter, nonblinded, randomized, controlled trial involving more than 6,000 patients that assessed 90-day mortality between those who received IIT (serum glucose goal range of 81-108 mg/dL) and conventional therapy (serum glucose concentration goal of 180 mg/dL or lower) in both SICU and MICU patients.7 The mortality rate was significantly higher in patients receiving IIT versus those given conventional therapy (27.5% vs. 24.9%, P = .02). However, IIT demonstrated a benefit in cardiac-surgery patients.13
A subgroup analysis of cardiac-surgery patients from the 2001 Leuven Surgical Trial revealed that ICU mortality decreased by 60% (from 5.1% to 2.1%) in conventional-therapy patients and in-hospital mortality decreased by 56% (from 7.5% to 3.4%) in IIT patients. IIT also improved morbidity through earlier weaning of mechanical ventilation and resulted in shorter SICU and hospital stays, lower incidences of acute renal failure and critical-illness polyneuropathy, reduced need for RBC transfusions, reduced inflammation, and lower cumulative Therapeutic Intervention Scoring System-28 with nonproblematic hypoglycemic episodes.
According to the Society of Thoracic Surgeons’ blood glucose guideline, glycemic control differs slightly between diabetic and nondiabetic patients. The Joint Commission requires that postoperative cardiac-surgery patients have tighter glucose control of <200 mg/dL in order to reduce postoperative complications such as infections necessitating antibiotics, need for breathing support, need for blood transfusions, and risk of acute renal failure.14 Controlling serum glucose concentrations <180 mg/dL in diabetic patients during cardiac surgery may reduce mortality, morbidity, incidence of wound infections, and hospital length of stay and may enhance long-term survival.2 Intraoperative glycemic control is not necessary in diabetic patients whose serum glucose concentrations remain <180 mg/dL, whereas intraoperative glycemic control is recommended in diabetic patients with higher serum glucose concentrations.
Glycemic Management
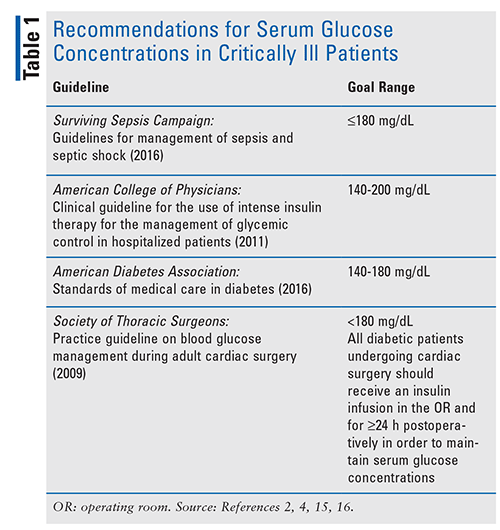
The American College of Physicians’ 2011 clinical practice guideline for the use of intensive insulin therapy for the management of glycemic control in hospitalized patients recommends a blood glucose target of 140 mg/dL to 200 mg/dL in critically ill adult patients once insulin is started.15 The 2016 American Diabetes Association’s Standards of Medical Care in Diabetes recommends a blood glucose target of 140 mg/dL to 180 mg/dL once insulin is started.16 The Society of Thoracic Surgeons’ blood glucose guideline recommends that perioperative patients with persistent blood glucose concentrations >180 mg/dL be started on insulin and maintain a serum glucose <180 mg/dL.2 There is no conclusive target range in the management of hyperglycemia in critically ill patients. However, strict management with IIT with goal serum glucose concentrations between 80 mg/dL and 110 mg/dL should be avoided due to risks of hypoglycemia and increased mortality. A summary of the guideline recommendations is given in TABLE 1.2,4,15,16
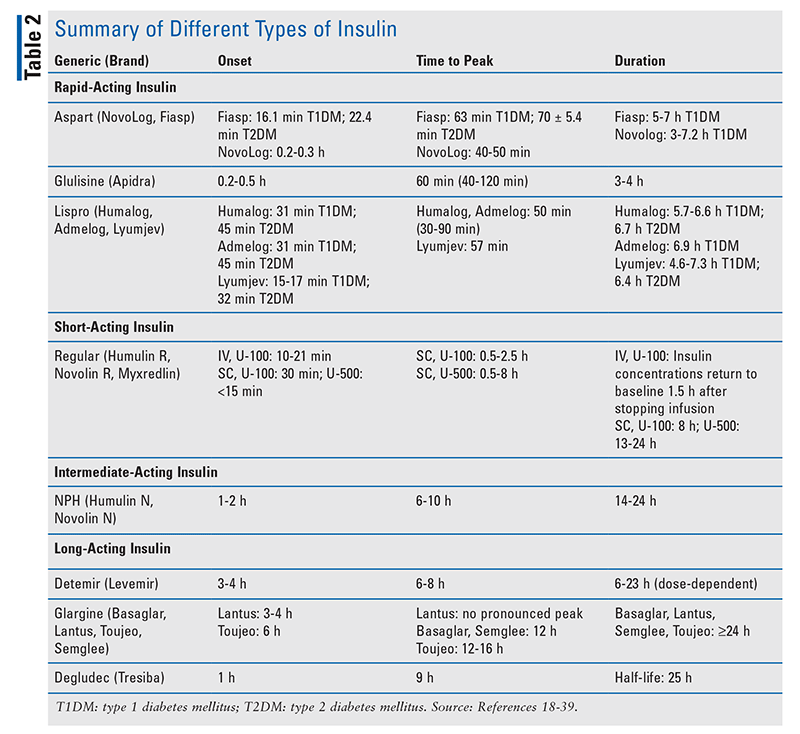
Types of Insulin: Glycemic control in the ICU is best achieved with continuous insulin infusions using regular human insulin rather than intermittent SC insulin injections or intermittent IV insulin boluses.2,14 IV insulin therapy permits rapid titration, enabling easier manipulation under certain circumstances, such as malabsorption, insulin deficiency, and resistance. The absorption of intermediate-acting insulin or combination intermediate- and rapid-acting insulin preparations is poor, making it difficult to lower blood glucose in patients with type 2 diabetes mellitus.17 See TABLE 2 for a summary of different insulins.18-39
Adverse Drug Effects: The use of IIT in critically ill patients is associated with a higher risk of hypoglycemia. Prolonged hypoglycemia in the ICU can lead to a number of complications, including death. Some evidence shows that there is an increased risk of mortality and extended length of hospital stay in patients who experienced at least one hypoglycemic event in the MICU, as well as an increased risk of dementia in diabetic patients.2 When blood glucose falls to <60 mg/dL, patients will start to experience sympathetic and parasympathetic symptoms, such as tachycardia, palpitations, sweating, nausea, and hunger.40 As blood glucose concentrations continue to drop to <50 mg/dL, neuroglycopenia symptoms, such as blurred vision and trouble speaking, will occur.40 Once blood glucose drops to <40 mg/dL, there is a high risk of coma, seizures, and even death. The NICE-SUGAR trial demonstrated that tight glucose control was linked to an increased incidence of death in critically ill patients. Based on these findings, the American Diabetes Association and the American College of Physicians decided that they would no longer support tight glucose control in the inpatient setting for critically ill patients on account of the lasting severe adverse effects of hypoglycemia.40
Transitions of Care: When patients are ready to be discharged from the ICU, they should be transitioned to an SC basal (glargine or detemir) or bolus (lispro, aspart, glulisine) insulin dosing schedule (TABLE 2). Daily insulin requirements can be estimated by extrapolating the amount of insulin required in the preceding 24 hours and considering the patient’s current nutritional intake.2 According to the American Association of Clinical Endocrinologists, the preprandial target serum glucose concentration should be <110 mg/dL, whereas a postprandial target serum glucose concentration of <180 mg/dL should be achieved in the stepdown units. Diabetic patients who were previously taking oral diabetes medications may be restarted on those agents once the glucose goals have been reached and a regular diet is being consumed.
Conclusion
Hyperglycemia in critically ill patients has been shown to be a significant cause of morbidity and mortality in patients admitted to the ICU. Although patients are managed based on hospital-specific glucose ranges, no standard range has been found to have the highest rate of survivability in the ICU patient population. When ICU patients are treated with IIT, the risk of hypoglycemia remains. In the acute-care setting, the pharmacist’s input is essential when management of pharmacokinetics-altering insulin may provoke symptoms of hypoglycemia that may result in additional adverse effects. Upon discharge, diabetic patients may continue their use of insulin, and to improve glycemic control nondiabetic patients with hyperglycemia may be started on insulin requiring basal and/or bolus dosing. Pharmacists in acute-care and ambulatory-care settings are able to adjust insulin therapy and educate patients about hypoglycemia or hyperglycemia in order to optimize patient outcomes.
REFERENCES
1. Hsu CW. Glycemic control in critically ill patients. World J Crit Care Med. 2012;1(1):31-39.
2. Lazar HL, McDonnell M, Chipkin SR, et al. The Society of Thoracic Surgeons practice guideline series: blood glucose management during adult cardiac surgery. Ann Thorac Surg. 2009;87(2):663-669.
3. Yao RQ, Ren C, Wu GS, et al. Is intensive glucose control bad for critically ill patients? A systematic review and meta-analysis. Int J Biol Sci. 2020;16(9):1658-1675.
4. Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449-461.
5. Eakins J. Blood glucose control in the trauma patient. J Diabetes Sci Technol. 2009;3(6):1373-1376.
6. Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359-1367.
7. NICE-SUGAR Study Investigators. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283-1297.
8. Thau L, Gandhi J, Sharma S. Physiology, cortisol. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2021.
9. Wieser V, Moschen AR, Tilg H. Inflammation, cytokines and insulin resistance: a clinical perspective. Arch Immunol Ther Exp (Warsz). 2013;61(2):119-125.
10. Morigny P, Houssier M, Mouisel E, Langin D. Adipocyte lipolysis and insulin resistance. Biochimie. 2016;125:259-266.
11. Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125-139.
12. Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377.
13. Vanhorebeek I, Ingels C, Van den Berghe G. Intensive insulin therapy in high-risk cardiac surgery patients: evidence from the Leuven randomized study. Semin Thorac Cardiovasc Surg. 2006;18(4):309-316.
14. Surgical Care Improvement Project. Cardiac surgery patients with controlled 6 A.M. postoperative blood glucose. https://manual.jointcommission.org/releases/archive/TJC2010B/MIF0056.html. Accessed October 11, 2021.
15. Qaseem A, Humphrey LL, Chou R, et al. Use of intensive insulin therapy for the management of glycemic control in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2011;154(4):260-267.
16. Standards of Medical Care in Diabetes—2016: summary of revisions. Diabetes Care. 2016;39(suppl 1):S4-S5.
17. Friedberg SJ, Lam YW, Blum JJ, Gregerman RI. Insulin absorption: a major factor in apparent insulin resistance and the control of type 2 diabetes mellitus. Metabolism. 2006;55(5):614-619.
18. Heise T, Pieber TR, Danne T, et al. A pooled analysis of clinical pharmacology trials investigating the pharmacokinetic and pharmacodynamic characteristics of fast-acting insulin aspart in adults with type 1 diabetes. Clin Pharmacokinet. 2017;56(5):551-559.
19. Pieber TR, Svehlikova E, Brunner M, et al. Fast-acting insulin aspart in people with type 2 diabetes: earlier onset and greater initial exposure and glucose-lowering effect compared with insulin aspart. Diabetes Obes Metab. 2019;21(9):2068-2075.
20. Kapitza C, Nosek L, Schmider W, et al. Single-dose euglycemic clamp study demonstrating pharmacokinetic and pharmacodynamic similarity between SAR341402 insulin aspart and US- and EU-approved versions of insulin aspart in subjects with type 1 diabetes. Diabetes Technol Ther. 2020;22(4):278-284.
21. Fiasp (insulin aspart) package insert. Plainsboro, NJ: Novo Nordisk Inc; December 2019.
22. NovoLog (insulin aspart) package insert. Plainsboro, NJ: Novo Nordisk Inc; March 2021.
23. Apidra (insulin glulisine) package insert. Bridgewater, NJ: sanofi-aventis U.S. LLC; December 2020.
24. Humalog (insulin lispro) package insert. Indianapolis, IN: Lilly USA, LLC; November 2019.
25. Admelog (insulin lispro) package insert. Bridgewater, NJ: sanofi-aventis U.S. LLC; December 2020.
26. Lyumjev (insulin lispro-aabc) package insert. Indianapolis, IN: Eli Lilly and Co; August 2021.
27. Humulin R (insulin human) package insert. Indianapolis, IN: Lilly USA, LLC; November 2019.
28. Novolin R (insulin human) package insert. Plainsboro, NJ: Novo Nordisk Inc; November 2019.
29. Myxredlin (insulin human) package insert. Deerfield, IL: Baxter Healthcare Corp; June 2020.
30. Humulin N (isophane insulin human) package insert. Indianapolis, IN: Lilly USA, LLC; November 2019.
31. Novolin N (isophane insulin human) package insert. Plainsboro, NJ: Novo Nordisk Inc; November 2019.
32. Levemir (insulin detemir) package insert. Plainsboro, NJ: Novo Nordisk Inc; March 2020.
33. Basaglar (insulin glargine) package insert. Indianapolis, IN: Eli Lilly and Co; July 2021.
34. Lantus (insulin glargine) package insert. Bridgewater, NJ: sanofi-aventis U.S. LLC; January 2021.
35. Toujeo (insulin glargine) package insert. Bridgewater, NJ: sanofi-aventis U.S. LLC; December 2020.
36. Semglee (insulin glargine) package insert. Morgantown, WV: Mylan Specialty LP; June 2020.
37. Tresiba (insulin degludec) package insert. Plainsboro, NJ: Novo Nordisk Inc; November 2019.
38. Plank J, Bodenlenz M, Sinner F, et al. A double-blind, randomized, dose-response study investigating the pharmacodynamic and pharmacokinetic properties of the long-acting insulin analog detemir. Diabetes Care. 2005;28(5):1107-1112.
39. Heinemann L, Linkeschova R, Rave K, et al. Time-action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care. 2000;23(5):644-649.
40. Pasala S, Dendy JA, Chockalingam V, et al. An inpatient hypoglycemia committee: development, successful implementation, and impact on patient safety. Ochsner J. 2013;13(3):407-412.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





