US Pharm. 2009;34(5)(Diabetes suppl):3-6.
Approximately 24 million people in the United States have type 2 diabetes, including 6 million who remain undiagnosed.1 Diabetes is associated with multiple complications, but cardiovascular disease (CVD) accounts for the majority of premature morbidity and mortality. People with diabetes are 2 to 4 times more likely than those without diabetes to die from CVD.2 Consequently, the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) recognizes diabetes as a CVD risk equivalent and recommends aggressive treatment of dyslipidemia to decrease the risk of CVD.3
Dyslipidemia is a major risk factor for the development of CVD, yet only 8% of patients with diabetes acknowledge correction of dyslipidemia as an important means of decreasing CVD risk.1 The CDC estimates that dyslipidemia is prevalent in 70% to 97% of patients with diabetes.4 The primary target of dyslipidemia management in both the nondiabetes and diabetes populations is low-density lipoprotein cholesterol (LDL-C). The dyslipidemia picture in patients with type 2 diabetes, however, manifests as an increased concentration of small, dense LDL-C; decreased high-density lipoprotein cholesterol (HDL-C); and elevated triglycerides (TG). The purpose of this article is to review the management of hypertriglyceridemia in the diabetes population.
ETIOLOGY
Hypertriglyceridemia is present in approximately one-third of the general population and in more than half of patients with diabetes.5 Patients with hyperinsulinemia (e.g., metabolic syndrome) or diabetes develop elevated TG from an overproduction of very-low-density lipoprotein (VLDL) and/or impaired TG lipolysis. The increased production of fatty acids by adipose cells results in the packing of this excess into VLDL, which is transported out of the liver. Also, patients with insulin resistance have less effective lipoprotein lipase, which is essential for breaking down TG in the circulation.
The larger clinical picture is the effect of hypertriglyceridemia on CVD risk in patients with diabetes. Although the NCEP classifies hypertriglyceridemia as an independent risk factor for CVD, a correlation does not exist after adjustment for LDL-C or HDL-C. Studies suggest, however, that patients with elevated TG and depressed HDL-C are at considerably higher risk for CVD owing to the presence of highly atherogenic lipoproteins.6 Paradoxically, this is often the clinical picture in patients with type 2 diabetes, indicating the need for a treatment approach that focuses on lifestyle interventions and pharmacologic therapy.
TREATMENT
Lifestyle Interventions
The most recent American Diabetes Association (ADA) recommendations suggest a focus on lifestyle interventions.7 Mild-to-moderate weight loss can significantly reduce TG levels by 22% and increase HDL-C by 9%.8 In addition, weight loss lowers blood glucose, thus helping improve TG. These metabolic changes also decrease the level of small, dense LDL-C particles by approximately 40%, thereby bettering the common atherogenic lipid profile associated with type 2 diabetes.8
Diet and exercise, the cornerstones of weight loss, can effectively lower TG levels. Excessive caloric intake not utilized by the body is converted to TG, which are stored in adipose tissue. Therefore, lower caloric intake and increased exercise help prevent this conversion and result in lower TG levels. The ADA does not recommend a specific diet plan, but it suggests that each patient's diet be individualized. General recommendations include restricting saturated fat, increasing monounsaturated fat, and reducing carbohydrate intake. In patients with significant hypertriglyceridemia (≥1,000 mg/dL), dietary fat should be limited to less than 10% of caloric intake.8 Alcohol generally should be avoided because of its ability to elevate TG levels. Thirty minutes of moderately intense aerobic exercise on most days of the week may reduce TG and increase HDL-C.8
Glycemic Control
Improvement of glycemic control can be extremely effective for reducing TG levels in patients with elevated TG and type 2 diabetes. Glucose-lowering medications can significantly reduce TG because, consequently, less glucose is available for conversion to TG. Insulin therapy has been shown to decrease TG levels by 50% while also lowering hemoglobin A1c (HbA1c) by 3%.2 Metformin can reduce TG by approximately 15% because of its ability to improve insulin resistance.2 Thiazolidinediones, including pioglitazone and rosiglitazone, activate the peroxisome proliferator-activated receptor (PPAR), but have varying effects on the lipid profile. Both pioglitazone and rosiglitazone increase HDL-C; pioglitazone, however, appears to decrease TG and have a neutral effect on LDL-C, while rosiglitazone increases LDL-C and has a neutral effect on TG. Rosiglitazone has been associated with a 30% to 40% increased risk of myocardial infarction in several meta-analyses, according to the ADA.9 The Prospective Pioglitazone Clinical Trial in Macrovascular Events concluded that pioglitazone did not affect CVD outcomes compared with placebo. Further meta-analyses suggest that pioglitazone may have a beneficial effect on CVD risk.9 Based on the available data, the ADA Consensus Statement currently advises against the use of rosiglitazone.9
Pharmacologic Therapies
See TABLE 1 for a brief summary of the following drug therapies for hypertriglyceridemia.
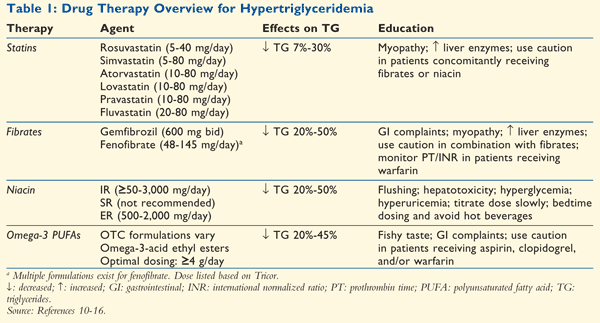
Hydroxymethyl Glutaryl Coenzyme A Reductase Inhibitors (Statins): LDL-C reduction remains the primary target of lipid management in all patients. Statin monotherapy is an essential component of diabetes care because significant evidence exists that statins reduce the risk of CVD in patients with diabetes. Statins can lower TG by 7% to 30%, with increased effectiveness at moderate-to-high doses, and can increase HDL-C by 5% to 15%.10 The importance of statin therapy in the diabetes population cannot be underestimated. Since statins are used primarily to lower LDL-C, further discussion will center on therapies used mainly to lower TG.
Fibric Acid Derivatives (Fibrates): Fibrates, namely gemfibrozil and fenofibrate, are the drugs of choice for hypertriglyceridemia. Fibrates activate PPARs, which lower TG levels by reducing TG synthesis in the liver through increased fatty-acid oxidation. This action decreases the TG content of VLDL particles. Gemfibrozil's mechanism of action is not understood as well as that of fenofibrate, but is thought to be similar. Both gemfibrozil and fenofibrate lower TG levels by 20% to 50%. Gemfibrozil generally has a neutral effect on LDL-C. Fenofibrate lowers LDL-C by 5% to 20% when used as monotherapy; little additional lowering of LDL-C occurs when it is combined with a statin, however. Fibrates can increase HDL-C by 10% to 35%, but the response is slightly better with fenofibrate.10 Both gemfibrozil and fenofibrate are taken orally, but fenofibrate is favored because it is given once daily compared with twice daily for gemfibrozil. Gemfibrozil is metabolized by the liver, and it is a CYP3A4 substrate and a strong inhibitor of CYP2C9 and CYP2C19. Fenofibrate is minimally metabolized in the liver, which limits its potential for drug-drug interactions.10
The side-effect profiles of gemfibrozil and fenofibrate are similar, for the most part. Fibrates are associated with an increased risk of myopathy and rhabdomyolysis, especially when used in combination with statins. The National Lipid Association (NLA) Statin Safety Task Force recently concluded that the use of gemfibrozil in combination with statins should generally be avoided based on data suggesting an increased risk of rhabdomyolysis.11 Gastrointestinal symptoms such as nausea, vomiting, diarrhea, and abdominal pain are common with fibrates, but become more tolerable over time. Hepatic function should be monitored closely, especially when fibrates are used in combination with statins. Fenofibrate has the potential to increase serum creatinine by approximately 12%, but this is generally reversible upon discontinuation or dose reduction. The National Kidney Foundation recommends gemfibrozil in patients with renal transplants or with chronic renal failure and elevated TG.11 The NLA Statin Safety Task Force, however, recommends decreasing the dose of gemfibrozil or fenofibrate in patients with a glomerular filtration rate (GFR) under 60 mL/minute (min)/1.73 m2 and avoiding the use of these drugs in patients with a GFR under 15 mL/min/1.73 m2.11
Fibrate therapy is often questioned because of limited outcome data, especially in the diabetes population and when used in combination with a statin. The Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial compared gemfibrozil with placebo in 2,531 patients (627 with diabetes). A 24% reduction in mortality from CVD, nonfatal myocardial infarction, or stroke was seen in the subgroup of diabetes patients; however, subsequent analyses found that the majority of benefit was due to the increase in HDL-C levels.12 In addition, the Fenofibrate Intervention and Event Lowering in Diabetes study failed to find a significant reduction in nonfatal myocardial infarction and fatal coronary heart disease in patients with type 2 diabetes.13 Studies are being conducted to determine whether fibrate monotherapy and fibrate-statin combination therapies have a positive effect on cardiovascular and mortality outcomes.
Niacin: Niacin (vitamin B3) has been used for decades to treat lipid abnormalities. Niacin decreases the production of VLDL and decreases the release of free fatty acids from adipose tissue in the blood. As a result, niacin lowers TG by 20% to 50% and raises HDL-C by 15% to 35%.10 LDL-C may be decreased by 5% to 25%, but this is dose-dependent, and many patients cannot tolerate the higher doses required for a noticeable reduction.10 Niacin's positive lipid effects, however, make it a viable option for patients with diabetic dyslipidemia.
Niacin is available in three formulations: immediate-release (IR), sustained-release (SR), and extended-release (ER). Most OTC niacin is IR, which is effective but requires tedious dose titration to prevent side effects (e.g., flushing). SR niacin increases the risk of hepatic dysfunction and generally should be avoided; ER niacin is associated with less flushing and a lower risk of hepatic dysfunction. Some OTC products claim to be "flush-free," but these products should be avoided: The active ingredient in these products is inositol hexanicotinate, which is thought not to enter the bloodstream, and clinical data proving its ability to lower cholesterol levels are lacking.
Flushing is a major adverse effect that often limits the use of niacin. Slow dose titration and the use of aspirin taken 30 minutes before the niacin may improve tolerability. Hepatic function should be monitored in patients taking niacin, especially when it is used concomitantly with a statin or fibrate.11 Probably the most controversial concern surrounding niacin use in the diabetic population is its ability to increase insulin resistance, which in turn may increase blood glucose levels. However, a 16-week study found no significant difference in HbA1c levels with 1,000 mg ER niacin, and an increase of only 0.3% with the 1,500-mg dose. Four patients in this study discontinued the niacin because of worsened glucose control.2 Several other studies have concluded that niacin has a limited effect on glucose levels.4 Nevertheless, the blood glucose levels of patients with diabetes who are started on niacin should be monitored for noticeable changes.
Omega-3 Polyunsaturated Fatty Acids (PUFAs): Docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) are essential fatty acids with antiarrhythmic, antilipid, antihypertensive, and antithrombotic properties. EPA and DHA are converted to prostaglandins, which are essential for cell regulation and cardiovascular function. Because of the high incidence of CVD in the diabetes population, omega-3 PUFAs are an attractive option for treating elevated TG.
Omega-3 PUFAs lower TG levels 20% to 45% by decreasing the production of VLDL and stimulating the oxidation of fatty acids.10 Patients with TG levels exceeding 500 mg/dL often derive the most benefit. The challenge in using omega-3 PUFAs for elevated TG is ensuring that the correct dose is taken. Noticeable changes in TG levels can be observed at 2 g/day, but a dose of 4 or more g/day provides significantly greater reductions.14 Most OTC products contain about 300 mg EPA/DHA per capsule, requiring at least 6 to 12 capsules daily to achieve the effective dose. A few OTC products contain higher doses of EPA/DHA; one example is Kirkland Signature Enteric Coated Fish Oil Concentrate, which contains 684 mg EPA/DHA per capsule.
Lovaza (omega-3-acid ethyl esters) is FDA-approved and contains 840 mg EPA/DHA per capsule, making it the most potent source of omega-3 PUFAs available. Patients can achieve significant reductions in TG levels by taking 2 to 4 capsules per day, but the eased pill burden comes with a significant price. Lovaza is often nonpreferred on prescription insurance plans and can cost $100 to $200 per month depending on the number of capsules required.15
Potential adverse effects of omega-3 PUFAs include potential exposure to environmental toxins, increased risk of bleeding, fishy aftertaste, burping, and upset stomach. Environmental toxins are a concern, but a recent analysis by Consumer Reports determined that 16 leading brands had no signs of contamination.16 Patients receiving antithrombotic therapy (i.e., warfarin, clopidogrel, aspirin) should be warned about the potential increased risk of bleeding when these agents are taken with omega-3 PUFAs. Patients who have experienced previous major bleeds, such as gastrointestinal or intracranial bleeding, may not be suitable candidates. Fishy aftertaste and occurrence of upset stomach can be minimized by taking the capsules with food. Another option is to store the capsules in the refrigerator or freezer, but Lovaza should be stored only at room temperature.15 The large size of fish oil capsules is a common complaint, especially in patients with dysphagia.
CLINICAL MANAGEMENT
Due to insufficient mortality and morbidity data, the decision to initiate pharmacologic treatment for hypertriglyceridemia is not without controversy. The use of combination therapy is associated with an increased risk of adverse events. To reduce the risk of pancreatitis, TG-lowering therapy should be strongly considered in patients with TG exceeding 400 mg/dL. TG-lowering therapy is required for severely elevated TG (≥1,000 mg/dL) because it is unlikely that lifestyle modifications and moderate- or high-dose statins will effectively lower TG sufficiently to reduce the risk of pancreatitis.17
The NCEP ATP III recommends the use of additional lipid-lowering therapies to achieve the non-HDL (total cholesterol minus HDL-C) goal when TG levels exceed 200 mg/dL. The non-HDL goal is 30 mg/dL higher than the LDL-C goal and is a reasonable secondary target after the LDL-C goal has been achieved.3 The clinician must consider the risks and benefits of achieving both the LDL-C goal and the non-HDL goal, as combination therapy often is needed to achieve this.
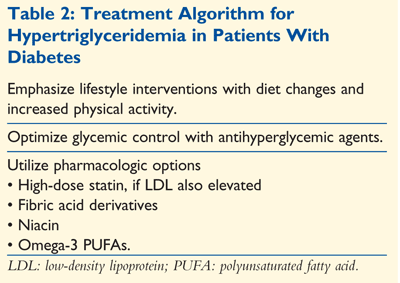
The management of hypertriglyceridemia in patients with diabetes varies little from that in patients without diabetes. Lifestyle interventions and improvement in glycemic control can significantly reduce TG levels in the diabetes population. Until more supportive evidence-based data are available, TG-lowering therapies should be reserved for patients at risk for pancreatitis or at very high risk for cardiovascular disease. See TABLE 2 for a general algorithm.
PHARMACIST'S ROLE
Patients with diabetes often want to know what TG are and how they affect their risk of heart disease and pancreatitis. Pharmacists in many settings have opportunities to educate patients about TG, and they can offer advice about nonpharmacologic methods of improving TG levels. Diabetes education regarding diet and exercise is key to improving glucose control and, consequently, lowering TG levels in this population.
Pharmacists should counsel patients receiving TG-lowering therapies regarding the potential for adverse effects, especially with fibrate-statin combination therapy. Patients with diabetes should be frequently reminded that they are at increased risk for CVD and that, often, multiple lipid-lowering therapies are needed to achieve lipid goals, especially the TG goal.
REFERENCES
1. National Diabetes Information Clearinghouse. National Diabetes Statistics, 2007. NIH Publication No. 08-3892. Bethesda, MD: National Diabetes Information Clearinghouse; June 2008.
2. Moon YS, Kashyap ML. Pharmacologic treatment of type 2 diabetic dyslipidemia. Pharmacotherapy. 2004;24:1692-1713.
3. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227-239.
4. Fagot-Campagna A, Rolka DB, Beckles GL, et al. Prevalence of lipid abnormalities, awareness, and treatment in U.S. adults with diabetes [abstract]. Diabetes. 2000;49(suppl 1):A78.
5. Jacobs MJ, Kleisli T, Pio JR, et al. Prevalence and control of dyslipidemia among persons with diabetes in the United States. Diabetes Res Clin Pract. 2005;70:263-269.
6. Krentz AJ. Lipoprotein abnormalities and their consequences for patients with type 2 diabetes. Diab Obes Metab. 2003;5(suppl 1):S19-S27.
7. Executive summary: standards of medical care in diabetes--2009. Diabetes Care. 2009;32(suppl 1):S6-S12.
8. Brunzell JD. Hypertriglyceridemia. N Engl J Med. 2007;357:1009-1017.
9. Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. Diabetes Care. 2009;32:1-11.
10. Ballantyne CM, O'Keefe JH, Gotto AM Jr. Dyslipidemia Essentials. 3rd ed. Birmingham, MI: Physicians' Press; 2007:1-175.
11. Davidson M, Armani A, McKenney JM, Jacobson T. Safety considerations with fibrate therapy. Am J Cardiol. 2007;99(suppl 1):S3-S-18.
12. Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. N Engl J Med. 1999;341:410-418.
13. Keech A, Simes RJ, Barter P, Best J, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849-1861.
14. Oh RC, Lanier JB. Management of hypertriglyceridemia. Am Fam Physician. 2007;75:1365-1371.
15. Lovaza (omega-3-acid ethyl esters) package insert. Research Triangle Park, NC: GlaxoSmithKline; November 2008.
16. Omega-3 oil: fish or pills? Consumer Reports. July 1, 2003.
17. American Diabetes Association. Dyslipidemia management in adults with diabetes. Diabetes Care. 2004;27(suppl 1):S68-S71.
To comment on this article, contact rdavidson@jobson.com.





