US Pharm. 2009;34(6):HS-1-HS-4.
Widespread administration of immunizations in the United States have helped eradicate such diseases as smallpox and poliomyelitis and can help prevent other serious illnesses and complications. Studies have shown that immunizations in adults aged 65 years and older reduce outpatient visits, hospitalizations, and deaths.1,2 Despite this, immunizations in adults continue to be underutilized. Each year, 50,000 U.S. adults die from vaccine-preventable diseases or their complications.3,4 Influenza kills approximately 36,000 people in the U.S. annually, with 90% of deaths occurring in those over the age of 65; pneumonia accounts for 11,000 to 14,000 deaths each year.3,4 Combined, pneumonia and influenza are the eighth leading cause of death in this country.4
Factors that contribute to underutilization of immunizations include lack of awareness about vaccine-preventable diseases, concern over safety and adverse reactions, and missed opportunities by health care professionals (HCPs).5-7 To increase awareness, programs such as Healthy People 2000 and Healthy People 2010 have been implemented. Health objectives set by Healthy People 2010 include increasing the proportion of noninstitutionalized adults who are appropriately vaccinated against pneumococcal disease and influenza.8 One significant goal is to "prevent disease, disability, and death from infectious diseases including vaccine preventable diseases."8 The target coverage for influenza and pneumococcal vaccines by 2010 is 90%.8
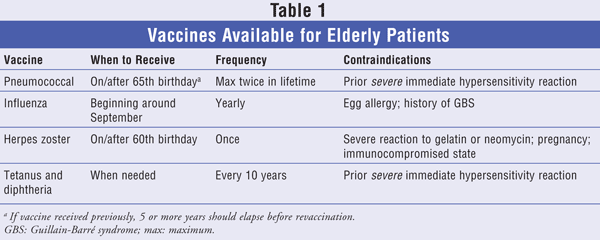
While pneumococcal and influenza vaccinations are emphasized to a greater degree owing to the number of deaths caused annually by these preventable infections, there are other vaccinations that older adults should receive (TABLE 1). These include one dose of zoster after age 60 and a tetanus/diphtheria booster every 10 years. It is imperative to recognize the importance of immunizations in all populations because, without widespread coverage, preventable infections could take more lives and the diseases we have worked so hard to eradicate could return.
Types of Vaccines
Pneumococcal Vaccine: Streptococcus pneumoniae is the causative bacterium in more than 25% of the 1 million elderly adults who contract community-acquired pneumonia each year.9 This organism also can lead to invasive diseases such as bacteremia and meningitis. Pneumococcal disease causes more fatalities than any other vaccine-preventable nonviral disease.10 Because of this, successful prevention of this disease has been a priority for more than 30 years.9
Currently, Pneumovax 23, the inactivated pneumococcal polysaccharide vaccine (PPV), is indicated for all persons aged 65 and older.10 PPV is a 23-valent vaccine that protects against 23 of the more than 80 serotypes of pneumococcal bacteria.11 This vaccine can be administered intramuscularly (IM) or subcutaneously (SC) in a 0.5-mL dose. Older adults should receive a one-time dose on or after their 65th birthday; however, in certain high-risk groups (including patients with asplenia, diabetes, or asthma/chronic obstructive pulmonary disease) who received the vaccine previously, at least 5 years should elapse before revaccination. Pneumovax 23 has been shown to be 75% effective against invasive infections in immunocompetent individuals over the age of 65. Adverse effects are rare, but if they occur usually include local injection-site reaction and low-grade fever.12
Influenza Vaccine: The influenza A virus causes a highly contagious respiratory infection affecting 5% to 20% of U.S. residents each year.13 Common symptoms include fever, muscle aches, chills, fatigue, and dry cough. After injection of the inactivated influenza vaccine, the host's body responds by producing antibodies to the different strains of virus included in the vaccine. The vaccine cannot cause infection by the influenza virus. In past years, the strains included in the vaccine were not the predominant strains of virus infecting the U.S. population; however, if an individual is infected by a strain that is different from but related to those in the vaccine, the antibodies that were created will attempt to fight the infection, thus making the disease course less severe.14
Although two types of influenza vaccine exist, only the trivalent inactivated influenza vaccine (TIV; various trade names) is appropriate for adults over the age of 65. (The live intranasal influenza vaccine [FluMist] is approved only for healthy persons aged 2 to 49 years.15) Because all seniors are at high risk for influenza, they should receive this vaccine annually. TIV, consisting of a 0.5-mL dose, is given as an IM injection in the deltoid muscle of the upper arm. Vaccines usually become available in September, and the first influenza outbreaks generally occur in October. Since peak influenza season is usually not until January or February, vaccination should occur throughout the entire season. TIV side effects are rare and typically include local injection-site reaction, low-grade fever, and body aches. Patients who should not receive the influenza vaccination include those with a true allergy to eggs and those who developed Guillain-Barré syndrome after a previous influenza vaccination. Individuals who have a severe illness with fever should wait until the illness subsides to be vaccinated.12
Herpes Zoster Vaccine: Herpes zoster, also known as shingles, develops upon reactivation of a latent varicella-zoster (chickenpox) viral infection of the sensory ganglia.16 About 1% of people over the age of 65 develop it each year.17 Shingles occurs when the virus, which has been dormant in the patient's nerve cells since the patient had chickenpox, starts to reproduce. It is a painful neuropathic condition, and the pain may continue even after the infection is gone.17 It is believed that decreased immune response renders older people more susceptible to infection with herpes zoster.16 The rate of herpes zoster infections is higher in the elderly population and in immunocompromised patients. If elderly patients can boost their immune response to the varicella-zoster virus, there should accordingly be a reduction in the number of people developing shingles.
Serious adverse events are rare with the zoster vaccine; however, 1 in 3 recipients experiences an injection-site reaction after administration.18 Headache occurs in slightly more than 1% of patients. Individuals who develop symptoms or signs of an allergic reaction, such as a significantly high fever or mood or behavior changes, should contact their HCP or seek emergency care.
Adults over the age of 60 should receive a single SC 0.65-mL dose of Zostavax, a live attenuated virus vaccine. Patients who are allergic to gelatin or neomycin, both of which are in the vaccine, should not be given the vaccine. Since the vaccine is a live virus and capable of causing infection, it should not be administered to a patient with an immune system weakened by a disease or drug treatment. According to the Shingles Prevention Study, the effectiveness of Zostavax ranges from 64% in people aged 60 to 69 years to 18% in those over the age of 80. The average overall effectiveness of the vaccine is 51%.18
Zostavax was in short supply at the end of 2008 and was backordered to most prescribers and pharmacies, but the manufacturer is once again making and shipping the vaccine.19
Tetanus and Diphtheria Vaccine: Tetanus (lockjaw) is an infectious disease caused by the bacterium Clostridium tetani, which attacks the nervous system. C tetani enters the body through an area of skin breakdown, such as a wound.20 Early symptoms of tetanus include locking of the jaw and muscle stiffness. In the early stage of the disease, the stiffness occurs primarily in the neck and abdomen; as a result, the patient may have difficulty opening his or her mouth or swallowing. As the disease progresses, more generalized, sustained muscle contractions--including generalized tonic-clonic seizurelike activity--occur.20 Death occurs in approximately 10% to 20% of tetanus cases, and the risk of death is higher in the elderly population.20 Prior to the existence of the vaccine, the U.S. averaged more than 1,300 cases per year.21 Since the vaccine's introduction, tetanus rates have dropped more than 96%.21
Diphtheria is an infectious disease of the respiratory system that is caused by the bacterium Corynebacterium diphtheriae.20 It is spread from person to person via airborne droplets when an infected person coughs or sneezes. The patient may initially notice a sore throat and a low-grade fever. If left untreated, diphtheria can lead to airway obstruction, breathing difficulty, heart failure, paralysis, coma, and death. Prior to development of the vaccine, the U.S. reported more than 175,000 diphtheria cases per year, but the number of cases has declined more than 99.9% since widespread vaccination.21
Tetanus and diphtheria toxoids adsorbed (Td; Decavac) is an inactivated vaccine that is indicated for primary vaccination in previously unimmunized patients aged 7 years or older and for routine booster immunization. Primary vaccination with two 0.5-mL doses of Td given 4 to 6 weeks apart and a third dose given 6 to 12 months later provide protection for at least 10 years. To ensure that a patient is protected, a 0.5-mL booster dose of Td should be given every 10 years after the primary vaccination.21 More frequent administration of booster doses may be associated with an increased incidence and severity of adverse reactions.20 In October 2005, the Advisory Committee on Immunization Practices voted to recommend replacing one booster dose of Td with tetanus, diphtheria, and acellular pertussis (Tdap; Adacel) in individuals aged 19 to 64 years. Tdap is not recommended for adults over the age of 64 owing to a lack of safety and immunogenicity studies.22
The majority of patients experience no adverse effects after receiving Td. Some patients report mild effects such as redness, tenderness, or swelling at the injection site. These effects usually occur within 2 days after vaccination and last for a couple of days. Rarely, serious allergic reactions or deep, aching pain and muscle wasting in the upper arm may occur; these effects typically present within 4 weeks of injection and can persist for months. A patient should contact his or her physician if a serious reaction occurs. The vaccine is contraindicated in patients who have experienced anaphylaxis or other serious allergic reactions following a previous dose of Td or any other tetanus or diphtheria toxoid-containing vaccine.23,24
Travel
Although travel by automobile is predominant in this population, some older adults who are in good physical health may travel internationally.25 It is important that these patients are up-to-date with all required immunizations. Depending on the area of travel, additional vaccinations may be necessary. An HCP can direct these patients to the CDC's Web site for travelers, which lists specific immunizations needed for international travel, as well as HCPs who can administer them.26
Conclusion
Because vaccine-preventable diseases remain a leading cause of death in the older population, it is the responsibility of the HCP to encourage these patients to become immunized (TABLE 1). The HCP is in a prime position to endorse immunizations to these patients so that their full benefit can be understood. Pharmacists are included in this challenge. Since many elderly patients are taking chronic medications, the pharmacist may be one of the first members of the health care team to be accessed and should therefore be ready for the encounter.
REFERENCES
1. Ashby-Hughes B, Nickerson N. Provider endorsement: the strongest cue in prompting high-risk adults to receive influenza and pneumococcal immunizations. Clin Excell Nurse Pract. 1999;3:97-104.
2. Nichol KL, Goodman M. The health and economic benefits of influenza vaccination for healthy and at-risk persons aged 65 to 74 years. Pharmacoeconomics. 1999;16(suppl 1):63-71.
3. National Foundation for Infectious Diseases. Facts about adult immunization.
www.nfid.org/pdf/factsheets/
4. National Center for Health Statistics. Healthy People 2000 Review, 1998-1999. Hyattsville, MD: Centers for Disease Control and Prevention; 1999. DHHS Publication No. (PHS) 99-1256.
5. CDC. Reasons reported by Medicare beneficiaries for not receiving influenza and pneumococcal vaccinations--United States, 1996. MMWR. 1999;48:556-890.
6. Findlay PF, Gibbons YM, Primrose WR, et al. Influenza and pneumococcal vaccination: patient perceptions. Postgrad Med J. 2000;76:215-217.
7. Morgan R, King D, Turnbull C. Influenza vaccination: do the aged reap the benefit? Postgrad Med J. 1995;71:22-23.
8. US Department of Health and Human Services. Healthy People 2010: Understanding and Improving Health. 2nd ed. Washington, DC: US Government Printing Office; November 2000.
9. Hak E, Sanders EA, Verheij TJ, et al. Rationale and design of CAPITA: a RCT of 13-valent pneumococcal vaccine efficacy among older adults. Neth J Med. 2008;66:378-383.
10. CDC. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices. MMWR. 1999;46:1-24.
11. Atkinson WL, Kroger AT. Pneumococcal polysaccharide vaccine (PPSV). CDC answers your questions.
www.immunize.org/catg.d/p2015.
12. Pneumovax 23 (pneumococcal vaccine polyvalent) package insert. Whitehouse Station, NJ: Merck and Co, Inc; January 2009.
13. CDC. Influenza: the disease.
www.cdc.gov/flu/about/disease/ index.htm. Accessed December 26, 2008.
14. CDC. Questions & answers about the 2008-2009 flu season.
www.cdc.gov/flu/2008-09_flu_
15. CDC. Influenza vaccination: a summary for clinicians.
www.cdc.gov/flu/professionals/
16. Levin MJ, Oxman MN, Zhang JH, et al. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis. 2008;197:825-835.
17. Schmader K. Herpes zoster in older adults. Clin Infect Dis. 2001;32:1481-1486.
18. Zostavax (zoster vaccine live) package insert. Whitehouse Station, NJ: Merck & Co, Inc; December 2008.
19. Merck Vaccines supply status.
www.merck.com/e-business/
20. CDC. Diphtheria, tetanus, and pertussis: recommendations for vaccine use and other preventative measures: recommendations of the Immunization Practices Advisory Committee (ACIP). MMWR.
21. CDC. Vaccines & immunizations: ACIP recommendations.
www.cdc.gov/vaccines/pubs/ 1991;40:1-28.
22. Adacel (tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine adsorbed) package insert. Swiftwater, PA: Sanofi Pasteur Inc; January 2009.
23. Decavac (tetanus and diphtheria toxoids adsorbed) package insert. Swiftwater, PA: Sanofi Pasteur Inc; December 2008.
24. CDC. Vaccines and immunizations: possible side-effects from vaccines.
www.cdc.gov/vaccines/vac-gen/
25. Teaff JD, Turpin T. Travel and the elderly. BNET News Publications.
http://findarticles.com/p/
26. CDC. Travelers' health.
http://wwwn.cdc.gov/travel/. Accessed February 9, 2009.





