US Pharm. 2016;41(7):47-50.
ABSTRACT: Zika virus, a growing global public-health concern, is primarily transmitted by infected Aedes species mosquitoes and has been associated with microcephaly in infants whose mothers were infected during pregnancy. Prevention of Zika virus is imperative, as there is currently no vaccine or specific treatment. In addition to practicing exposure avoidance and using physical barriers, persons at risk should also apply topical mosquito repellents and insecticides for optimal protection. DEET, IR3535, oil of lemon eucalyptus, and picaridin are the insect repellents recommended by the CDC. It is important to advise patients to follow label instructions and to reapply the product as directed for maximal efficacy and safety. Permethrin may be sprayed on gear and clothing for extra protection.
Not only are their bites an uncomfortable nuisance, but mosquitoes are vectors for West Nile virus, chikungunya virus, dengue, and malaria.1 Most recently, concerns have centered on the mosquito-transmitted Zika virus. Zika virus was identified in Uganda in 1947, with the first human cases reported between 1952 and 1953. In May 2015, the Pan American Health Organization issued a notification about the first Zika case in Brazil; subsequently, the World Health Organization deemed Zika virus a Public Health Emergency of International Concern.2-5 By March 2016, the virus had spread to 33 countries and American territories. Although hundreds of travel-associated cases of Zika virus have been reported, no local mosquito-borne cases have been identified in the United States to date. The local spread of the virus in the U.S. may result from a rise in cases contracted outside the U.S. and then brought into the country.3,6 Today, Zika virus is a growing global public-health concern and a challenge for patients and providers alike.
Zika Virus
Zika virus is primarily transmitted by an infected Aedes species mosquito. These mosquitoes tend to bite mainly in the daytime, unlike other species, which are predominant at dusk. It is difficult to control Aedes species because they require only small amounts of water to reproduce and their eggs can survive harsh conditions. Transmission also has occurred from expectant mother to fetus, as well as via sexual contact with an infected male partner.3,7,8 Zika virus is of special concern for pregnant women and women of childbearing age because of its association with the microcephaly epidemic. In late 2015, Brazilian authorities reported a possible association between Zika infection during pregnancy and the occurrence of microcephaly (small head size for gestational age and sex), which involves brain-growth abnormalities, in their infants. The greatest risk of microcephaly occurs during the first trimester of pregnancy, and further investigation is ongoing.5,8,9
The incubation period for Zika virus is unknown, but is estimated to be <1 week. Most infected individuals are asymptomatic, and it is estimated that <20% of those infected display symptoms. Common clinical symptoms include maculopapular rash (90%), fever (65%), joint pain (65%), and conjunctivitis (55%). The rash is pruritic, and the fever is typically short-term and low-grade; myalgia and headache are also possible. These symptoms are generally mild and can last several days to a week. Additionally, an association with the more severe complication of Guillain-Barré syndrome has been reported.5,8,10 Prevention of Zika virus is imperative, and pharmacists—especially those in ambulatory settings—play a major role in recommending appropriate prevention strategies to those at risk.11
Clinical Management
The CDC provides guidance and resources for the prevention, diagnosis, and treatment of Zika virus.12 Additionally, clinicians should be aware that the National Library of Medicine also has resources regarding the virus.13
Blood tests are available that can diagnose Zika virus during the first week after symptom onset. Testing facilities were initially limited, which resulted in longer-than-desirable wait times for results.14,15 However, the FDA has granted an Emergency Use Authorization for the first test from a commercial laboratory in order to allow testing to become more broadly available.16 The treatment of confirmed Zika virus—a detailed discussion of which is beyond the scope of this article—generally consists of supportive care, such as rest, fluids, and the use of analgesics and antipyretics. If a patient experiences the previously discussed symptoms prior to confirmatory testing, acetaminophen—which is safe to use during pregnancy—is recommended to relieve fever and pain. Because of the risk of hemorrhage, aspirin, products containing aspirin, and other nonsteroidal anti-inflammatory drugs (e.g., ibuprofen) should not be recommended until dengue is ruled out.17 No vaccine or preventable drug is currently available; nevertheless, agencies are actively working to accelerate research into potential treatments and vaccines.18
Therefore, the prevention of Zika virus, with measures aimed at reducing infections in pregnant women, is crucial. Exposure avoidance (avoiding unnecessary travel to at-risk areas, refraining from unprotected sexual contact with at-risk partners, and limiting outdoor activity during peak mosquito-biting hours) and physical barriers (i.e., window and door screens, full-coverage clothing, air conditioning, and bed nets), as well as the removal of standing water in which mosquitoes can breed, are all strategies to prevent Zika virus infection. However, topical mosquito repellents and insecticides are required for optimal protection. Pharmacists should be aware of the different products available and their unique properties and be able to educate the patient on the appropriate use of the selected product.11,19,20
Products for Zika Virus Prevention
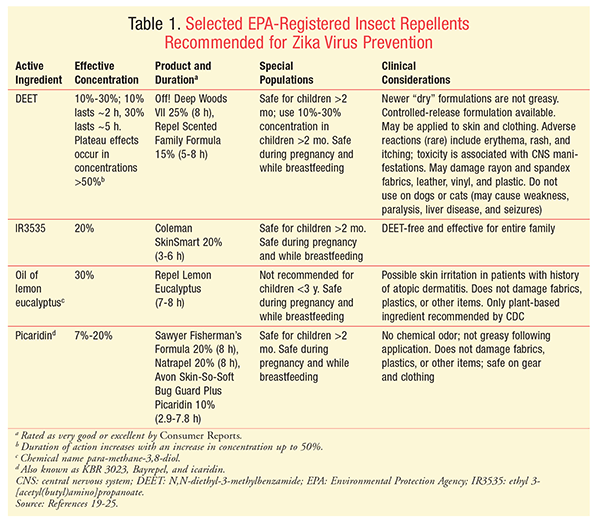
Topically Applied Repellents: Environmental Protection Agency (EPA)–registered repellents that are recommended by the CDC for Zika virus prevention include DEET (N,N-diethyl-3-methylbenzamide), IR3535 (ethyl 3-[acetyl(butyl)amino]propanoate), oil of lemon eucalyptus, and picaridin (TABLE 1).19-25 Catnip oil, oil of citronella, and 2-undecanone are registered, but not recommended. All registered products are assigned a registration number, which is listed on the product label. Registered repellents are safe and effective for human use when label instructions are followed.26 Effective concentrations of active ingredients (TABLE 1) are associated with a longer duration. In some cases, a product containing a lower concentration of the active ingredient may be effective but have a shorter duration of action. Some lower-concentration products may last only minutes, rather than hours, and most have been rated poor to fair overall.22 Additionally, the efficacy and duration of a product may vary according to the ambient temperature, level of activity, amount of perspiration, exposure to water, mosquito species, and product formulation (e.g., controlled-release or not). If a bite occurs sooner than expected, reapplication is advised.19
Examples of ingredients in unregistered repellents include cedar oil, citronella oil (used in products meeting the requirements for exemption), geranium oil, peppermint oil, and soybean oil. Unregistered agents (e.g., essential oils) are generally safe and pose minimal risk to humans, but have not been evaluated for effectiveness.26,27 Products commonly contain more than one ingredient, provide protection from minutes to approximately 1 hour, and are commonly rated overall as fair.22 In most cases, essential oils are safe for human use; however, oils can be toxic and can cause irritation when they are applied at high concentrations.28
The third category of agents listed by the EPA is classified as illegal because the products are neither registered nor exempt. If a product does not have an EPA registration number and is not listed on the EPA’s minimum-risk pesticide website,26 it is likely illegal. However, the EPA cautions that it may be difficult to determine the status of certain products.26
There are a multitude of commercially available devices that may be used in addition to repellents, but a device alone should not be relied on for complete protection. Devices include wristbands; lighter-ignited coils, sticks, torches, and candles; electronic zappers; carbon dioxide traps; and portable fans and diffusers.19
Permethrin: In addition to a repellent, permethrin may be sprayed on gear and clothing for extra protection, but permethrin should not be applied directly to skin. Permethrin, a synthetic chemical based on a natural extract from the chrysanthemum flower, is classified as a repellent and insecticide. Items such as backpacks, boots, and sleeping bags must be pretreated 24 to 48 hours in advance and allowed to dry. Items should be re-treated according to label instructions.19 Unfortunately, permethrin resistance in mosquitoes has been reported.21
Practical Recommendations and Patient Counseling
DEET, IR3535, oil of lemon eucalyptus, and picaridin should be recommended to patients for Zika virus prevention.11 Although DEET has generally been the most effective topical repellent, picaridin may be better tolerated on the skin.29 Patients should not rely solely on alternative products or devices for full protection.21,22,30 The EPA has a website that helps patients select an appropriate insect repellent.31 The use of an EPA-registered and CDC-recommended insect repellent on the skin should be encouraged for improved protection, as should the application of permethrin to other items. Important patient counseling points for product use, application, and safety include 1) the agent’s duration of action and need for reapplication; 2) application of sunscreen 15 minutes before the repellent, if needed (DEET may decrease sun-protective factor, and sunscreen-and-repellent combinations should be avoided); 3) instructions to spray the product in the palm of the hand and then apply it to the face; 4) the importance of not applying repellents to skin beneath clothing; 5) awareness of the product’s expiration date; 6) caution with use in young children; and 7) damage of plastics and fabrics by some products.11,19,32 One of the biggest communication challenges remains the education of the approximately 70% of patients who believe that repellent products currently on the market are not safe.22
Case Study: Recommending Mosquito Repellents for Zika Prevention
A family of five (father, mother, 5-year-old, 2-year-old, and mother’s 25-year-old married sister) are planning a summer evening picnic at a local lake. The father will fish during the day and then join the family for dinner. A nice playground for the children is located close to the water. What recommendations for a mosquito repellent can you give the family?
The use of an effective concentration of DEET or picaridin plus permethrin is widely recommended.21 An effective concentration of IR3535 plus permethrin would also be appropriate.
A 25% DEET-containing product (e.g., Off! Deep Woods VII) applied every 6 to 8 hours would be appropriate for the entire family. Products with an adequate concentration of DEET have a history of safety and are economical. The product should be reapplied sooner if a bite occurs. Alternatively, other products from TABLE 1 are appropriate, excluding oil of lemon eucalyptus, which is not recommended for children aged <3 years.33
In addition, a product containing permethrin should be sprayed on shoes, boots, shirts, and hats 24 to 48 hours before the outdoor activity. Special consideration should be given to women of childbearing age (mother and mother’s sister). Effective concentrations of products containing DEET, IR3535, oil of lemon eucalyptus, and picaridin are safe and effective for use in pregnancy or while breastfeeding if used according to label instructions. Women of childbearing age should apply a repellent and reapply according to their level of activity. If an infant aged <2 months were in attendance, he or she should be protected with netting, long-sleeved clothing, or a hat because EPA-registered products are not recommended for children aged <2 months.26 Additionally, if a pet dog were present, DEET should not be used on the dog.25
The adults should be advised to use a product according to the label instructions, to note the expiration date, and to check the label for the concentration of active ingredient. If the concentration is not listed on the label, another EPA-registered agent should be selected.
Conclusion
Zika virus is a growing threat, especially for travelers and for women who are pregnant or may become pregnant. Pharmacists can contribute to prevention of the virus by recommending appropriate insect repellents and providing patient education. DEET, IR3535, oil of lemon eucalyptus, and picaridin are the commonly available EPA-registered insect repellents recommended by the CDC for prevention. The appropriate use and application of the selected product is imperative for optimal efficacy and safety.
REFERENCES
1. National Institute for Occupational Safety and Health. Mosquito-borne diseases. www.cdc.gov/niosh/topics/outdoor/mosquito-borne/default.html. Accessed April 27, 2016.
2. CDC. About Zika virus disease. www.cdc.gov/zika/about/index.html. Accessed April 27, 2016.
3. Paixão ES, Barreto F, da Glória Teixeira M, et al. History, epidemiology, and clinical manifestations of Zika: a systematic review. Am J Public Health. 2016;106:606-612.
4. World Health Organization. Zika: the origin and spread of a mosquito-borne virus. www.who.int/bulletin/online_first/16-171082/en. Accessed April 27, 2016.
5. Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika virus. N Engl J Med. 2016;374:1552-1563.
6. CDC. Areas with Zika. www.cdc.gov/zika/geo/index.html. Accessed April 27, 2016.
7. CDC. Transmission & risks. www.cdc.gov/zika/transmission/index.html. Accessed April 27, 2016.
8. CDC. Clinical evaluation & disease. www.cdc.gov/zika/hc-providers/clinicalevaluation.html. Accessed April 27, 2016.
9. CDC. For pregnant women. www.cdc.gov/zika/pregnancy/index.html. Accessed April 27, 2016.
10. CDC. Symptoms, diagnosis, & treatment. www.cdc.gov/zika/symptoms/index.html. Accessed April 27, 2016.
11. CDC. Prevention. www.cdc.gov/zika/prevention/index.html. Accessed April 27, 2016.
12. CDC. For healthcare providers. www.cdc.gov/zika/hc-providers/index.html. Accessed April 27, 2016.
13. NLM Outreach and Specific Populations Branch. Zika virus health information resources. http://content.govdelivery.com/accounts/USNLMOS/bulletins/132b65d?reqfrom=share. Accessed April 27, 2016.
14. CDC. Diagnostic testing. www.cdc.gov/zika/hc-providers/diagnostic.html. Accessed April 27, 2016.
15. CDC. State & local public health laboratories. www.cdc.gov/zika/state-labs/index.html. Accessed April 27, 2016.
16. Zika test from Quest Diagnostics authorized by the FDA for emergency use. http://newsroom.questdiagnostics.com/2016-04-28-Zika-Test-from-Quest-Diagnostics-Authorized-by-the-FDA-for-Emergency-Use. Accessed May 2, 2016.
17. Health Alert Network. Recognizing, managing, and reporting Zika virus infections in travelers returning from Central America, South America, the Caribbean, and Mexico. http://emergency.cdc.gov/han/han00385.asp. Accessed April 27, 2016.
18. National Institute of Allergy and Infectious Diseases. NIAID research approach to Zika virus. www.niaid.nih.gov/topics/Zika/ResearchApproach/Pages/default.aspx. Accessed April 27, 2016.
19. CDC. Protection against mosquitoes, ticks, & other arthropods. Yellow Book. wwwnc.cdc.gov/travel/yellowbook/2016/the-pre-travel-consultation/protection-against-mosquitoes-ticks-other-arthropods. Accessed April 27, 2016.
20. CDC. Avoid mosquito bites. www.cdc.gov/features/stopmosquitoes. Accessed April 27, 2016.
21. Diaz JH. Chemical and plant-based insect repellents: efficacy, safety, and toxicity. Wilderness Environ Med. 2016;27:153-163.
22. Byrne S. Mosquito repellents that best protect against Zika. Consumer Reports. www.consumerreports.org/insect-repellents/mosquito-repellents-that-best-protect-against-zika. Accessed April 27, 2016.
23. American Academy of Pediatrics. 2016 summer safety tips. www.aap.org/en-us/about-the-aap/aap-press-room/news-features-and-safety-tips/pages/Summer-Safety-Tips.aspx. Accessed April 27, 2016.
24. DEET. Lexi-Drugs. Hudson, OH: Lexi-Comp, Inc; 2016. http://online.lexi.com. Accessed April 27, 2016.
25. Merck Veterinary Manual. Ectoparasiticides used in small animals. www.merckvetmanual.com/mvm/pharmacology/ectoparasiticides/ectoparasiticides_used_in_small_animals.html. Accessed April 27, 2016.
26. Environmental Protection Agency (EPA). Regulation of skin-applied repellents. www.epa.gov/insect-repellents/regulation-skin-applied-repellents. Accessed April 27, 2016.
27. EPA. Minimum risk pesticides exempted from FIFRA registration. www.epa.gov/minimum-risk-pesticides. Accessed April 28, 2016.
28. Rutledge CR, Day JF. Mosquito repellents. University of Florida/IFAS Extension. http://edis.ifas.ufl.edu/pdffiles/IN/IN41900.pdf. Accessed April 27, 2016.
29. Buff W, Fabel PH. Insect bites and stings and pediculosis. In: Krinsky DL, Ferreri SP, Hemstreet B, et al, eds. Handbook of Nonprescription Drugs: An Interactive Approach to Self-Care. 18th ed. Washington, DC: American Pharmacists Association; 2015.
30. Fradin MS, Day JF. Comparative efficacy of insect repellents against mosquito bites. N Engl J Med. 2002;347:13-18.
31. EPA. Find the insect repellent that is right for you. www.epa.gov/insect-repellents/find-insect-repellent-right-you. Accessed April 27, 2016.
32. EPA. Using insect repellents safely and effectively. www.epa.gov/insect-repellents/using-insect-repellents-safely-and-effectively. Accessed April 27, 2016.
33. FDA. Insect repellent use and safety in children. www.fda.gov/Drugs/EmergencyPreparedness/ucm085277.htm. Accessed April 27, 2016.
To comment on this article, contact rdavidson@uspharmacist.com.





