US Pharm. 2010;35(5)(Diabetes suppl):3-7.
In healthy adults, basal insulin concentrations of 5 to 15 µU/mL help to maintain fasting plasma glucose concentrations (FIGURE 1).1 Immediately following a meal, insulin concentration peaks at 60 to 80 µU/mL, returning to basal levels 1 to 3 hours later. In type 2 diabetes mellitus, progressive loss of beta cells results in the disruption of endogenous insulin secretion, in turn leading to requirement for insulin therapy. Considering that the daily pattern of normal insulin secretion is complex, close replication of this pattern is needed to address both fasting and prandial glucose control.
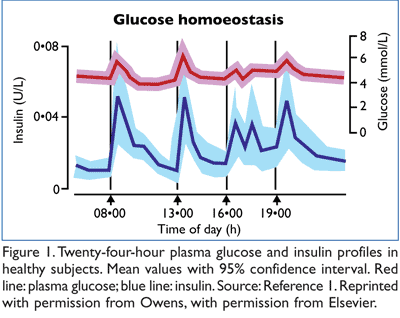
In human insulin preparations, such as regular human insulin (RHI), insulin molecules typically self-aggregate to form dimers, which in turn stabilize around zinc ions to form hexamers.2 Following injection, the subcutaneous insulin depot is diluted by the interstitial fluid, causing hexamers to break down into dimers and biologically active monomers. The dissociation of hexamers into dimers and monomers is a rate-limiting step in absorption for all insulins and contributes to the delay in the effect of RHI. Because insulin hexamers are too bulky to be transported across the vascular endothelium, there is a 30- to 60-minute lag phase between injection and onset of action, which requires careful dose administration and food consumption. In addition to slow onset, a slow clearance can result in prolonged periods of elevated insulin and “delayed” hypoglycemia.
Exogenous basal insulin delivery has traditionally involved single or twice-daily injections of neutral protamine Hagedorn (NPH) insulin, which is a formulation of protamine insulin in a zinc suspension. Protamine prolongs the absorption of NPH insulin, causing an intermediate duration of action (10-16 h), a significant peak in action (4-6 h), and an unpredictable profile of insulin absorption and activity.3,4 Hypoglycemia is a disadvantage of NPH insulin, particularly nocturnal hypoglycemia following bedtime injection.5
Pharmacokinetic/
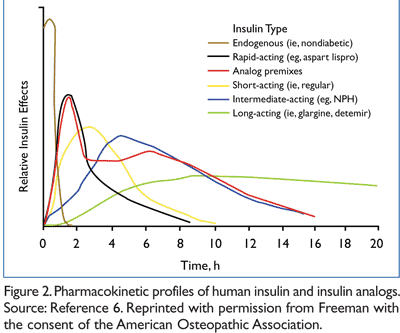
Rapid-Acting (Bolus) Insulin Analogs
Currently available rapid-acting insulin analogs (“bolus” insulins) include insulin lispro, insulin aspart, and insulin glulisine. Self-association of insulin molecules into dimers and hexamers is reduced by substitution of one or two key amino acids in insulin lispro, glulisine, and aspart. These structural modifications result in disruption of dimer formation and a 200- to 300-fold reduction in dimerization constants when compared to RHI.2,7 Lower dimerization constants allow for faster absorption and rapid onset of action compared to RHI.2
Monomeric insulin preparations are susceptible to deamidation, degradation, and fibril formation. In order to facilitate physical and chemical stability, zinc ions and phenolic ligands are added to these preparations.2 Studies comparing insulin lispro and insulin aspart formulations with and without zinc have shown no significant difference in rate of absorption and time-action profile.8,9
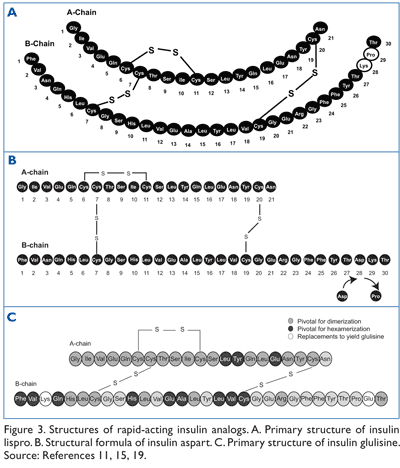
Insulin lispro is structurally similar to RHI except that the sequence has been reversed at positions B28 (proline) and B29 (lysine) to match the sequence of insulin-like growth factor 1 (IGF-1) (FIGURE 3A).10,11 This structural modification offers a distinct pharmacokinetic advantage over RHI because it allows for injection immediately before meals, thereby limiting postprandial blood glucose excursions.10,12 One study demonstrated that mean peak insulin action occurred at 99 ± 39 minutes for insulin lispro compared to 179 ± 93 minutes for RHI.13
Insulin aspart is formed by replacing proline at B28 with aspartic acid (FIGURE 3B).14,15 This structural alteration reduces self-association of monomers. Hexamers of insulin aspart dissociate rapidly to monomers that are more rapidly absorbed than RHI. Rapid disappearance from the injection site contributes to rapid onset of action. A study by Mudaliar et al showed that the time to peak action was 94 ± 46 minutes for insulin aspart, compared to 173 ± 62 minutes for RHI.16 Kang et al reported that 79% of the insulin aspart dose was absorbed in the first 3 hours of administration versus 51% of the same dose of RHI.17
Insulin glulisine is formed by replacing glutamic acid for lysine at B29 and replacing asparagine with lysine at B3 (FIGURE 3C).18,19 The exchange of the neutral, polar, and hydrophilic asparagine at B3 with basic, polar, and hydrophilic lysine and the exchange of basic lysine at B29 with acidic, polar, and hydrophilic glutamic acid results in an additional charge. This contributes to a slightly lower isoelectric point compared to RHI, which enhances solubility of insulin glulisine at physiologic pH. The onset of absorption of insulin glulisine was 20 to 30 minutes earlier compared with RHI.20
Although the plasma clearance of bolus insulin analogs is similar to RHI, the mean residence time is shorter, resulting in a more rapid onset of action (within 10-15 minutes of administration) and a shorter duration of action, thus improving their ability to control postprandial glucose.
Long-Acting (Basal) Insulin Analogs
There are two long-acting insulin analogs (“basal” insulins) commercially available in the United States: insulin glargine and insulin detemir.
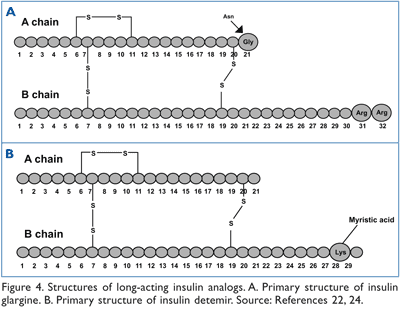
Insulin glargine has two arginine residues added to the C terminus of the B chain and glycine replacing an asparagine at A21 (FIGURE 4A).21,22 A shift in the isoelectric point (from pH 5.4 to pH 6.7) results in reduced solubility at physiologic pH. Insulin glargine forms amorphous microprecipitates at the neutral pH of the subcutaneous tissue and is slowly released to provide basal insulin supplementation. While NPH insulin produces a definite peak within 4 to 8 hours and returns to baseline by 16 hours, insulin glargine, at a much lower concentration, produces a plateau within 6 to 8 hours, which remains unchanged for up to 24 hours.3
Insulin detemir is a neutral, noncrystalline, clear, soluble preparation in which the lysine at B29 is covalently bound to myristic acid (FIGURE 4B).23,24 This modification causes insulin detemir molecules to self-associate and also bind to albumin. Increased self-association and binding to albumin contribute to protracted action and also reduction of biological variability. Prolonged residence time at the injection site and in the plasma bound to albumin delays distribution to the target tissues and achieves a constant metabolic effect over time.23 One study showed that the time-action profile and the duration of action of insulin detemir were comparable to that of insulin glargine, whereas within-subject variability was significantly lower.25
Clinical Effectiveness
Bolus Analogs
Bolus insulin regimens have demonstrated efficacy in controlling postprandial glucose. When compared with RHI insulin, rapid-acting analogs have a time-action profile similar to endogenous postprandial insulin release and, therefore, can be injected within 15 minutes before a meal, unlike RHI, which must be administered at least 30 minutes before a meal.26 This option offers flexibility to patients, especially to those with irregular meal schedules.
Postprandial rise in serum glucose levels was significantly lower following treatment with insulin lispro compared to RHI (30% lower at 1 h; and 53% lower at 2 h after a meal).12 Premixed combinations of insulin lispro with neutral insulin lispro protamine suspension in 25%/75% or 50%/50% combinations are significantly more effective in lowering postprandial blood glucose concentrations than premixed RHI plus NPH insulin at 30%/70%, and they more closely mimic physiologic insulin secretion.27
When combined with glyburide, the mean 2-hour postprandial glucose after a test meal was significantly lower for insulin lispro (10.87 ± 2.88 mmol/L) versus NPH insulin (12.21 ± 3.12 mmol/L) or metformin (12.72 ± 3.26 mmol/L).28 Similarly, glycosylated hemoglobin (HbA1C) was significantly lower for insulin lispro (7.68% ± 0.88%) compared to NPH insulin (8.51% ± 1.38%) or metformin (8.31% ± 1.31%).
Insulin aspart injected 30 minutes before a meal reduced postprandial blood glucose without the risk of hypoglycemia.29 Insulin aspart also improved postprandial glucose control compared to RHI when given immediately before a meal, as opposed to 30 minutes before a meal.30 In this study, 6-hour postprandial glucose values for insulin aspart and RHI were 899 versus 1102 mmol x min/L (P <0.01). When combined with NPH insulin, treatment with insulin glulisine resulted in a greater reduction in HbA1C when compared to RHI (0.46% vs. 0.30%).31
The rapid pharmacodynamic effects of bolus analogs are accompanied by a reduced risk of hypoglycemia. Meta-analyses representing several hundred patient-years demonstrated a 25% reduction in the occurrence of severe hypoglycemia with the use of insulin lispro compared to RHI.32 Insulin aspart was associated with lower incidence of major nocturnal hypoglycemia when compared with RHI.14 In a study demonstrating the noninferiority of insulin glulisine to RHI, rates of symptomatic and severe hypoglycemia were similar between the treatment groups; however, nocturnal symptomatic hypoglycemia was less with insulin glulisine when compared with RHI (9.1% vs. 14.5%; P =0.0290).33
All three rapid-acting analogs possess similar pharmacokinetic properties and have demonstrated similar safety and efficacy. Generally, they exhibit markedly improved absorption with peak plasma concentration of about 40 minutes compared to 100 minutes for RHI.2,13
Basal Analogs
Ideal basal insulins are peakless and long-lasting, with reproducible subcutaneous absorption. NPH insulin is not an ideal basal insulin because of its peak action profile and day-to-day variability in absorption, which is further accentuated by the need for resuspension prior to injection—a critical step often overlooked by patients, resulting in hyper- or hypoglycemia.34 Because insulin glargine is soluble in acidic conditions, it does not require resuspension prior to administration. In the case of insulin detemir, acylation of fatty acids to the insulin molecule allows for a neutral liquid preparation that does not precipitate at any stage in the administration-absorption process, thereby eliminating an important source of variability.
Long-acting insulin analogs result in reduced variability in blood glucose–lowering effects compared to NPH insulin.25,35 The absence of a clear peak and constant delivery of insulin over 24 hours contributes to the lower risk of nocturnal hypoglycemia and, therefore, to the ability to achieve lower fasting blood glucose levels when given before the evening meal or bedtime.
Despite similar improvement in HbA1C levels and fasting plasma glucose, lower postdinner glucose concentration (9.9 ± 0.2 vs. 10.7 ± 0.3 mmol/L) and lower incidence of nocturnal hypoglycemia were observed with treatment with insulin glargine compared to NPH insulin (9.9% vs. 24.0%).36 In a similar study, comparable changes in fasting plasma glucose and HbA1C levels were achieved in both insulin glargine and NPH insulin treatment groups; however, 25% more patients in the insulin glargine group attained glycemic control without experiencing nocturnal hypoglycemia.37
Seventy percent of patients receiving either insulin detemir or NPH insulin achieved HbA1C levels of ≤7.0%. Additionally, the proportion of patients without hypoglycemia was higher with insulin detemir than with NPH insulin (26% vs. 16%; P =0.008).38 Furthermore, compared with NPH insulin, treatment with insulin detemir reduced the risk for overall hypoglycemia by 47% (P <0.001) and for nocturnal hypoglycemia by 55% (P <0.001).
In another study, insulin detemir combined with oral antidiabetic drugs (OADs) reduced 24-hour and nocturnal hypoglycemic events by 65% (P =0.031) and 53% (P =0.019), compared to NPH insulin + OADs.39
In the PREDICTIVE trial, all insulin detemir treatment groups demonstrated significant reductions in HbA1C levels with no major hypoglycemic events and a combined average weight loss of 0.9 kg.40 In the GOAL A1C Study, when combined with OADs, insulin glargine produced significant reductions in HbA1C levels with low rates of hypoglycemia.41
Weight Gain
Concerns about weight gain are a well-recognized barrier to initiation or to intensification of insulin therapy. In several studies, consistent and significant favorable weight changes have been reported with insulin detemir when compared to NPH insulin.
Once-daily insulin detemir and insulin glargine were evaluated in an indirect comparison of published clinical trials with NPH insulin as a common comparator.42 Patients in the insulin detemir group gained significantly less weight. Data from the PREDICTIVE study demonstrated a significant decrease in body weight (-0.7 kg; P <0.0001) in the insulin detemir group.43 Almost 68% of the patients either did not gain weight or experienced weight reduction during 14 weeks of treatment.
The mechanism behind the weight-sparing property associated with insulin detemir is yet to be determined. One plausible theory is that patients are less likely to engage in “defensive snacking” in anticipation of possible hypoglycemia.
Conclusions
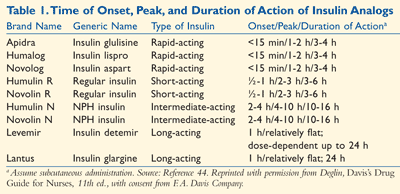
Insulin analogs have provided superior alternatives to conventional human insulins. The unique time/action profiles of insulin analogs are attributed to structural alterations at pivotal amino acid residues, resulting in more physiologic pharmacokinetic and pharmacodynamic profiles (TABLE 1).44 Bolus insulin analogs demonstrate rapid onset of action and quick absorption, and impart flexibility to the patient by allowing injection immediately prior to or after a meal. These analogs are associated with improved postprandial glycemic control and less risk of hypoglycemia. Basal analogs provide levels of glycemic control comparable to NPH insulin, while demonstrating a lack or reduction of peak activity, a longer duration of action, reduced variability, reduced nocturnal hypoglycemia (overall hypoglycemia in some trials), and reduced propensity for weight gain (with insulin detemir). Treatment regimens based on both rapid- and long-acting analogs have resulted in improvements in HbA1C levels, better glycemic control, and reduced hypoglycemia compared with treatments with regular insulin and NPH insulin.
Acknowledgement: The author wishes to thank Anu Santhanagopal, PhD, of DesignWrite LLC for providing writing and editorial assistance.
REFERENCES
1. Owens DR, Zinman B, Bolli GB. Insulins today and beyond. Lancet. 2001;358(9283):739-746.
2. Brange J, Volund A. Insulin analogs with improved pharmacokinetic profiles. Adv Drug Deliv Rev. 1999;35:307-335.
3. Heinemann L, Linkeschova R, Rave K, et al. Time-action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care. 2000;23:644-649.
4. Heinemann L. Variability of insulin absorption and insulin action. Diabetes Technol Ther. 2002;4:673-682.
5. Fanelli CG, Pampanelli S, Porcellati F, et al. Administration of neutral protamine Hagedorn insulin at bedtime versus with dinner in type 1 diabetes mellitus to avoid nocturnal hypoglycemia and improve control. A randomized, controlled trial. Ann Intern Med. 2002;136:504-514.
6. Freeman JS. Insulin analog therapy: improving the match with physiologic insulin secretion. J Am Osteopath Assoc. 2009;109:26-36.
7. Brems DN, Alter LA, Beckage MJ, et al. Altering the association properties of insulin by amino acid replacement. Protein Eng. 1992;5:527-533.
8. Wiefels K, Kuglin B, Hubinger A, et al. Insulin kinetics and dynamics in insulin-dependent diabetic patients after injections of human insulin or the insulin analogues X14 and X14+Zn. In: Berger M, Gries FA, eds. Frontiers in Insulin Pharmacology. Stuttgart, NY: Thieme Medical Publishers; 1993:97-101.
9. Luttermann JA, Pijpers E, Netten PM, et al. Glycaemic control in IDDM patients during one day with injection of human insulin or the insulin analogues insulin X14 and insulin X14 (+ Zn). In: Berger M, Gries FA, eds. Frontiers in Insulin Pharmacology. Stuttgart, NY: Thieme Medical Publishers; 1993:102-109.
10. Heller S. Insulin lispro: a useful advance in insulin therapy. Expert Opin Pharmacother. 2003;4:1407-1416.
11. Humalog (insulin lispro injection, usp) prescribing information. Indianapolis, IN: Eli Lilly and Company; 2007.
12. Anderson JH, Jr., Brunelle RL, Koivisto VA, et al. Improved mealtime treatment of diabetes mellitus using an insulin analogue. Multicenter Insulin Lispro Study Group. Clin Ther. 1997;19:62-72.
13. Howey DC, Bowsher RR, Brunelle RL, et al. [Lys(B28), Pro(B29)]-human insulin. A rapidly absorbed analogue of human insulin. Diabetes. 1994;43:396-402.
14. Chapman TM, Noble S, Goa KL. Insulin aspart: a review of its use in the management of type 1 and 2 diabetes mellitus. Drugs. 2002;62:1945-1981.
15. NovoLog (insulin aspart [rDNA origin] injection) prescribing information. Princeton, NJ: Novo Nordisk A/S; 2007.
16. Mudaliar SR, Lindberg FA, Joyce M, et al. Insulin aspart (B28 asp-insulin): a fast-acting analog of human insulin: absorption kinetics and action profile compared with regular human insulin in healthy nondiabetic subjects. Diabetes Care. 1999;22:1501-1506.
17. Kang S, Creagh FM, Peters JR, et al. Comparison of subcutaneous soluble human insulin and insulin analogues (AspB9, GluB27; AspB10; AspB28) on meal-related plasma glucose excursions in type I diabetic subjects. Diabetes Care. 1991;14:571-577.
18. Becker RH. Insulin glulisine complementing basal insulins: a review of structure and activity. Diabetes Technol Ther. 2007;9:109-121.
19. Apidra (insulin glulisine) prescribing information. Bridgewater, NJ: sanofi-aventis, LLC; 2008.
20. Becker RH, Frick AD. Clinical pharmacokinetics and pharmacodynamics of insulin glulisine. Clin Pharmacokinet. 2008;47:7-20.
21. Barnett AH. Insulin glargine in the treatment of type 1 and type 2 diabetes. Vasc Health Risk Manag. 2006;2:59-67.
22. Lantus (insulin glargine [rDNA origin] injection) prescribing information. Bridgewater, NJ: sanofi-aventis, LLC; 2007.
23. Chaykin LB. Insulin detemir and its unique mechanism of action. Internet J Endocrinol. 2007;4:1-13.
24. Levemir (insulin detemir [rDNA origin] injection) prescribing information. Princeton, NJ: Novo Nordisk; 2007.
25. Klein O, Lynge J, Endahl L, et al. Albumin-bound basal insulin analogues (insulin detemir and NN344): comparable time-action profiles but less variability than insulin glargine in type 2 diabetes. Diabetes Obes Metab. 2007;9:290-299.
26. Hirsch IB. Insulin analogues. N Engl J Med. 2005;352:174-183.
27. Giugliano D, Ceriello A, Razzoli E, et al. Defining the role of insulin lispro in the management of postprandial hyperglycaemia in patients with type 2 diabetes mellitus. Clin Drug Invest. 2008;28:199-210.
28. Bastyr EJ III, Stuart CA, Brodows RG, et al. Therapy focused on lowering postprandial glucose, not fasting glucose, may be superior for lowering HbA1c. Diabetes Care. 2000;23:1236-1241.
29. Gredal C, Rosenfalck AM, Dejgaard A, et al. Targeting postprandial hyperglycaemia in patients with recently diagnosed type 2 diabetes with a fixed, weight-based dose of insulin aspart. Scand J Clin Lab Invest. 2008;68:739-744.
30. Rosenfalck AM, Thorsby P, Kjems L, et al. Improved postprandial glycaemic control with insulin aspart in type 2 diabetic patients treated with insulin. Acta Diabetol. 2000;37:41-46.
31. Dailey G, Rosenstock J, Moses RG, et al. Insulin glulisine provides improved glycemic control in patients with type 2 diabetes. Diabetes Care. 2004;27:2363-2368.
32. Brunelle RL, Llewelyn J, Anderson JH Jr, et al. Meta-analysis of the effect of insulin lispro on severe hypoglycemia in patients with type 1 diabetes. Diabetes Care. 1998;21:1726-1731.
33. Rayman G, Profozic V, Middle M. Insulin glulisine imparts effective glycaemic control in patients with type 2 diabetes. Diabetes Res Clin Pract. 2007;76:304-312.
34. McAulay V, Frier BM. Insulin analogues and other developments in insulin therapy for diabetes. Expert Opin Pharmacother. 2003;4:1141-1156.
35. Heise T, Nosek L, Ronn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes. 2004;53:1614-1620.
36. Yki-Jarvinen H, Dressler A, Ziemen M, et al. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. Diabetes Care. 2000;23:1130-1136.
37. Dailey G, Riddle M, Benedetti MM, et al. A meta-analysis of phase III/IIIb studies comparing insulin glargine with human NPH insulin in type 2 diabetes: severe hypoglycemia is less common with glargine [abstract]. Diabetes. 2003;52(suppl 1):A444.
38. Hermansen K, Davies M, Derezinski T, et al. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care. 2006;29:1269-1274.
39. Philis-Tsimikas A, Charpentier G, Clauson P, et al. Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes. Clin Ther. 2006;28:1569-1581.
40. Meneghini L, Koenen C, Weng W, et al. The usage of a simplified self-titration dosing guideline (303 Algorithm) for insulin detemir in patients with type 2 diabetes—results of the randomized, controlled PREDICTIVE™ 303 study. Diabetes Obes Metab. 2007;9:902-913.
41. Kennedy L, Herman WH, Strange P, et al. Impact of active versus usual algorithmic titration of basal insulin and point-of-care versus laboratory measurement of HbA1c on glycemic control in patients with type 2 diabetes: The Glycemic Optimization with Algorithms and Labs at Point of Care (GOAL A1C) trial. Diabetes Care. 2006;29:1-8.
42. Fakhoury W, Lockhart I, Kotchie RW, et al. Indirect comparison of once daily insulin detemir and glargine in reducing weight gain and hypoglycaemic episodes when administered in addition to conventional oral anti-diabetic therapy in patients with type-2 diabetes. Pharmacology. 2008;82:156-163.
43. Dornhorst A, Luddeke HJ, Sreenan S, et al. Insulin detemir improves glycaemic control without weight gain in insulin-naive patients with type 2 diabetes: subgroup analysis from the PREDICTIVE study. Int J Clin Pract. 2008;62:659-665.
44. Deglin JH, Vallerand AH. Insulins and insulin therapy. In: Davis’s Drug Guide for Nurses. 11 ed. Philadelphia, PA: F.A. Davis Company; 2009:1379-1380.
To comment on this article, contact rdavidson@uspharmacist.com.





