US Pharm. 2019;44(1):HS2-HS6.
ABSTRACT: Malignant hyperthermia is a rare, life-threatening clinical syndrome of hypermetabolism involving the skeletal muscle. In susceptible individuals, this reaction is triggered primarily by exposure to volatile inhalational anesthetic agents and succinylcholine (a muscle relaxant). In patients who are susceptible to malignant hyperthermia, the ryanodine receptor in skeletal muscle is abnormal and causes a buildup of calcium in skeletal muscle, resulting in a massive metabolic reaction upon exposure to the triggering agents. Malignant hyperthermia must be treated rapidly in order to avoid a fatal outcome. Despite the rarity of malignant hyperthermia, healthcare facilities that use known triggering agents must be fully prepared to treat it.
Malignant hyperthermia (MH) is a pharmacogenic disorder of skeletal muscle. It manifests as a life-threatening hypermetabolic crisis associated with a rapid, uncontrolled increase in myoplasmic calcium in skeletal muscles. This hypermetabolic crisis is most often triggered in susceptible persons by the administration of volatile anesthetics and the neuromuscular blocking agent succinylcholine.1,2
MH susceptibility is an inherited autosomal-dominant trait.2 Individuals who are susceptible to MH have abnormal skeletal-muscle ryanodine receptors; this abnormality interferes with calcium regulation in the muscle. When an abnormal ryanodine receptor that controls calcium release is present, a buildup of calcium can occur, leading to a substantial metabolic reaction upon exposure to a triggering agent.3-5
Signs
In a patient experiencing MH, the reaction causes increased carbon dioxide production, metabolic and respiratory acidosis, accelerated oxygen consumption, heat production, sympathetic nervous system activation, hyperkalemia, and disseminated intravascular coagulation (DIC), all of which result in multisystem organ failure. Early clinical signs of MH are hypercapnia (elevated carbon dioxide levels in the blood), tachypnea, tachycardia, and muscle rigidity. Later signs may include hyperthermia, ECG changes related to hyperkalemia, and myoglobinuria.6
MH-susceptible individuals may not consistently develop the acute syndrome when exposed to anesthetics. An MH crisis can occur at first exposure to a triggering agent; however, the average MH-susceptible patient has had previous exposures before a documented reaction occurs.7 A history of uneventful anesthesia with MH-triggering agents should not rule out susceptibility to MH; MH episodes resulting from the use of supposedly safe agents have also been reported.
Diagnostic Testing
Testing for MH susceptibility is most useful in making treatment decisions for surgical patients who may be susceptible to MH. Diagnostic testing is not recommended as a screening tool for the general population. Diagnostic tests include the caffeine-halothane contracture test and genetic testing (ryanodine receptor gene sequencing). The muscle-contracture test, which is considered the gold standard, requires a skeletal-muscle biopsy from the patient’s thigh.8
Epidemiology
Studies show that MH complicates approximately one in 100,000 surgeries in adults and one in about 30,000 surgical procedures in children.2 The true incidence of MH susceptibility has not been precisely established in the United States because of the lack of universal reporting and the fact that many MH-susceptible persons have not been exposed to a triggering agent. MH occurs globally in all ethnic groups. Reactions are reported to occur more frequently in males than in females, and patients younger than 19 years account for approximately 50% of reported events.6,7
Triggering Agents
Through the years, a number of medications have been implicated as MH triggers. According to the Malignant Hyperthermia Association of the United States (MHAUS), the following agents approved for use in the U.S. are known triggers of MH: inhaled general anesthetics, halothane, desflurane, enflurane, ether, isoflurane, sevoflurane, and succinylcholine.9 Although the potent inhalation anesthetics are the principal triggers, there is strong evidence that modern agents can cause MH reactions in the same way that halothane does, in some cases with delayed-onset reactions occurring several hours into anesthesia.10
The vast majority of MH cases have occurred while a patient was receiving a volatile anesthetic agent with or without succinylcholine.6 MH has been reported after the administration of succinylcholine in the absence of inhaled anesthetic agents; the majority of these cases were in patients whose MH susceptibility was proved by biopsy.11
MH has also been reported in MH-susceptible individuals after exposure to heat stress or vigorous exercise. Rare cases of children developing spontaneous fatal MH under normal living conditions, with postmortem tests revealing abnormal ryanodine receptor mutations, have been reported.12,13
Presentation
MH may occur in the operating room or soon afterward, in the initial postoperative period. The presentation of MH varies, and most patients do not develop all of the signs. In a review of 255 MH events, the order of appearance of clinical signs, from earliest to latest, was as follows: masseter spasm, hypercarbia, sinus tachycardia, muscle rigidity, tachypnea, cyanosis, skin mottling, rapidly increasing temperature, elevated temperature, sweating, ventricular tachycardia, cola-colored urine, ventricular fibrillation, and excessive bleeding.6
The presentation of pediatric patients with acute MH varies by age. According to the North American Malignant Hyperthermia Registry, sinus tachycardia, hypercarbia, and rapid temperature increase were the most common signs of acute MH in patients 18 years and younger; these findings were also more common in patients aged 13 to 18 years (oldest cohort). Children aged 25 months to 12 years (middle cohort) experienced more masseter spasm, and those aged 0 to 24 months (youngest cohort) were more likely to develop skin mottling and were half as likely to develop muscle rigidity.14
It is a misconception that hyperthermia is the initial presenting sign of MH. Hyperthermia typically occurs later and is absent when the condition is first suspected. In the review of 255 MH cases, hyperthermia was one of the first signs in just 8.2% of crises and was the only initial sign in 3.9%.6 Fulminant MH reactions are rare; most cases are slower and display subtle changes. The MHAUS runs a 24-hour hotline that may be consulted for emergency assistance.
Treatment
Indications for treatment of MH include signs of hypermetabolism, rapid increase in carbon dioxide (metabolic acidosis may be delayed), tachycardia, and muscle or jaw rigidity. Patients may not present with all of these clinical signs, but without a persuasive alternative diagnosis, it is recommended that dantrolene be initiated and triggering agents be discontinued immediately rather than waiting too long to do so, which could lead to a negative outcome.1
According to the MHAUS, these four things should be done as soon as possible in treating an acute MH event15:
1. Notify the surgeon to terminate the procedure as soon as feasible and discontinue volatile agents and succinylcholine.
2. Obtain the dantrolene/MH cart; if at a surgical center rather than a hospital, call 911.
3. Hyperventilate the patient with 100% oxygen at 10 L/minute.
4. Administer dantrolene.
IV dantrolene is the only drug FDA-approved for the treatment of MH. Dantrolene is a hydantoin-derivative skeletal muscle relaxant that acts by directly interfering with the release of calcium from the sarcoplasmic reticulum. Evidence suggests that, during an MH reaction, triggering agents produce a change in the patient’s skeletal-muscle cells, resulting in elevated myoplasmic calcium; dantrolene is thought to prevent or reduce this increase, which activates the acute catabolic processes associated with MH.16 Dantrolene is an isotonic solution with a half-life of 4 to 11 hours. The initial dose recommended by the MHAUS is 2.5 mg/kg, with the dose continuously repeated until symptoms subside; large doses (>10 mg/kg) may be required in some patients, and it is suggested that an alternative diagnosis be considered if large doses of dantrolene do not resolve symptoms.
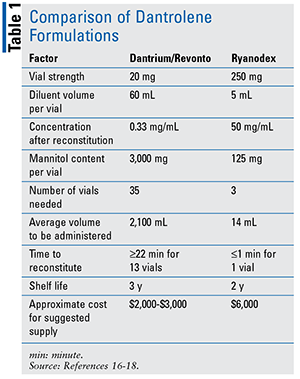
There are two available formulations of dantrolene. Dantrium and Revonto, the older formulation, provide 20 mg of dantrolene sodium/60 mL after reconstitution in sterile water. Dantrium and Revonto should be reconstituted by adding 60 mL of Sterile Water for Injection (without a bacteriostatic agent), and the vial should be shaken until the solution is clear. The newer formulation, Ryanodex, is an injectable suspension of dantrolene sodium that provides 250 mg of dantrolene sodium/5 mL after reconstitution. Each vial of Ryanodex should be reconstituted with 5 mL of Sterile Water for Injection (without a bacteriostatic agent) and shaken to ensure a uniform, opaque, orange-colored suspension. See TABLE 1 for a comparison of dantrolene formulations.16-18
It is recommended that blood gases be tested to determine the degree of metabolic acidosis, and the provider should consider administering sodium bicarbonate at a dosage of 1 to 2 mEq/kg for a base excess greater than –8, for a maximum dosage of 50 mEq. Patients with MH should be cooled if their core temperature is greater than 39°C or is rapidly rising; cooling should be stopped when the temperature is less than 38°C. Patients with hyperkalemia should be treated with calcium chloride 10 mg/kg (maximum dose 2,000 mg) or calcium gluconate (maximum dose 3,000 mg), sodium bicarbonate 1 to 2 mEq/kg IV (maximum dose 50 mEq), glucose, and insulin (pediatric patients, 0.1 U regular insulin/kg IV and 0.5 g/kg dextrose; adult patients, 10 U regular insulin IV and 50 mL 50% dextrose), and glucose levels should be checked hourly. In the case of refractory hyperkalemia, albuterol, kayexelate, dialysis, or extracorporeal membrane oxygenation should be considered if a patient has experienced cardiac arrest.15
Dysrhythmias should be treated with standard medications; however, calcium channel blockers must be avoided during an MH crisis because they can worsen hyperkalemia and hypotension. Patients having an MH reaction should be diuresed to greater than 1 mL/kg/hour urine output; if creatine kinase (CK) or potassium rises, myoglobinuria should be suspected and a bicarbonate infusion of 1 mEq/kg/hour should be initiated to alkalinize the urine. It is important to appropriately monitor patients experiencing an MH reaction, including monitoring of core temperature and urine output.15
Once the initial MH reaction is under control and the patient is stable, the patient should be continuously monitored in a post anesthesia care unit or ICU for at least 24 hours.17 Indications that a patient is stabilizing are normal or declining end-tidal carbon dioxide levels, decreased or stable heart rate with no signs of dysrhythmias, resolving hyperthermia, and no generalized muscular rigidity.15 Immediately following an acute MH reaction, dantrolene should be continued for at least 24 hours at a dosage of 1 mg/kg by IV injection every 4 to 6 hours or by IV infusion at a dosage of 0.25 mg/kg/hour.15-18 The MHAUS suggests that dantrolene can be discontinued or the dosing interval increased to up to every 12 hours if all of the following criteria are met: metabolic stability for 24 hours, core temperature less than 38°C, decreasing CK, no evidence of myoglobinuria, and no muscle rigidity. It is important that providers continuously monitor patients for MH-related complications, which include changes in consciousness, cardiac dysrhythmias and/or dysfunctions, pulmonary edema, renal dysfunction secondary to acute tubular necrosis, DIC, hepatic dysfunction, muscular weakness (rhabdomyolysis vs. effect of dantrolene), and compartment syndrome secondary to rhabdomyolysis.15
Preparation and Readiness
It is important that hospitals respond appropriately to cases of MH. The Centers for Medicare and Medicaid Services (CMS) released data in 2016 showing that, from 2011 through 2015, inspections at eight hospitals and health systems revealed deficiencies related to MH preparedness. Common MH-related issues encountered by health systems are 1) insufficient supply of dantrolene and 2) staff not properly trained in its administration.19
The MHAUS recommends that dantrolene be accessible within 10 minutes after the decision to treat is made and that at least 700 mg—enough to treat a 70-kg patient—be available; this equates to 35 20-mg vials or three 250-mg vials. The MHAUS also suggests storing, in or near the operating room, an MH cart containing the necessary medications for MH treatment: dantrolene; Sterile Water for Injection; five vials of 50 mL sodium bicarbonate (8.4%); two vials of 50 mL dextrose 50%; two vials of 10 mL calcium chloride (10%); one vial of regular insulin 100 U/mL; three prefilled syringes of lidocaine for injection (2%) 100 mg/5 mL or 100 mg/10 mL; and a minimum of 3,000 mL of refrigerated saline solution. It is also recommended that the MH cart contain all the required general equipment, monitoring equipment, nursing supplies, and laboratory testing supplies.20 The CMS does not provide a detailed policy on how health organizations will be surveyed on their ability to respond to an MH crisis, but it expects them to follow current standards of practice, which are outlined by the MHAUS.19
Conclusion
Although MH is a rare event complicating only about one in 100,000 surgeries, it can be deadly. MH-susceptible individuals often do not know that they are susceptible until an MH reaction occurs. Healthcare facilities using agents that can trigger MH—volatile anesthetics and succinylcholine—must be prepared to rapidly detect and initiate treatment for MH. MH can occur in the operating room or shortly afterward. The presentation of MH can vary, and many patients do not develop all of the signs. It is a misconception that hyperthermia is the initial presenting sign; it typically occurs later and therefore is not present when MH is first diagnosed. Once an MH event is suspected, the surgeon must be notified to stop the procedure immediately and discontinue volatile anesthetics and succinylcholine. The patient should be hyperventilated with 100% oxygen at 10 L/minute and IV dantrolene administered as soon as possible. It is imperative that staff who will respond to an MH reaction be familiar with the MHAUS website and its contents to ensure that their team is properly trained and their facility has the recommended treatment modalities readily available in order to achieve the best possible outcome.
REFERENCES
1. Gupta PK, Hopkins PM. Diagnosis and management of malignant hyperthermia. BJA Education. 2017;17(7):249-254.
2. Malignant Hyperthermia Association of the United States (MHAUS). Frequently asked questions: malignant hyperthermia. www.mhaus.org/faqs. Accessed October 16, 2018.
3. Wappler F. Malignant hyperthermia. Eur J Anaesthesiol. 2001;18(10):632-652.
4. MacLennan DH, Phillips MS. Malignant hyperthermia. Science. 1992;256(5058):789-794.
5. Lee-Chiong TL Jr, Stitt JT. Disorders of temperature regulation. Compr Ther. 1995;21(12):697-704.
6. Larach MG, Gronert GA, Allen GC, et al. Clinical presentation, treatment, and complications of malignant hyperthermia in North America from 1987 to 2006. Anesth Analg. 2010;110(2):498-507.
7. Rosenberg H, Davis M, James D, et al. Malignant hyperthermia. Orphanet J Rare Dis. 2007;2:21.
8. MHAUS. MHAUS guidelines: testing for malignant hyperthermia (MH) susceptibility: how do I counsel my patients? www.mhaus.org/testing/introduction-to-mh-testing/testing-for-malignant-hyperthermia-mh-susceptibility-how-do-i-counsel-my-patients. Accessed October 19, 2018.
9. MHAUS. Safe and unsafe anesthetics. www.mhaus.org/healthcare-professionals/be-prepared/safe-and-unsafe-anesthetics. Accessed October 16, 2018.
10. Hopkins PM. Malignant hyperthermia: pharmacology of triggering. Br J Anaesth. 2011;107(1):48-56.
11. Riazi S, Larach MG, Hu C, et al. Malignant hyperthermia in Canada: characteristics of index anesthetics in 129 malignant hyperthermia susceptible probands. Anesth Analg. 2014;118:381-387.
12. Lavezzi WA, Capacchione JF, Muldoon SM, et al. Case report: death in the emergency department: an unrecognized awake malignant hyperthermia-like reaction in a six-year-old. Anesth Analg. 2013;116(2):420-423.
13. Groom L, Muldoon SM, Tang ZZ, et al. Identical de novo mutation in the type 1 ryanodine receptor gene associated with fatal, stress-induced malignant hyperthermia in two unrelated families. Anesthesiology. 2011;115(5):938-945.
14. Nelson P, Litman RS. Malignant hyperthermia in children: an analysis of the North American Malignant Hyperthermia Registry. Anesth Analg. 2014;118(2):369-374H
15. MHAUS. Managing a crisis. www.mhaus.org/healthcare-professionals/managing-a-crisis. Accessed October 25, 2018.
16. Revonto (dantrolene) package insert. Louisville, KY: WorldMeds, LLC; October 2016.
17. Ryanodex (dantrolene) package insert. Woodcliff Lake, NJ: Eagle Pharmaceuticals, Inc; September 2017.
18. Dantrium (dantrolene) package insert. Chestnut Ridge, NY: Par Pharmaceutical; July 2016.
19. Traynor K. Readiness for malignant hyperthermia can be survey stumbling block. Am J Health Syst Pharm. 2016;73:852-853.
20. MHAUS. How to be prepared. www.mhaus.org/healthcare-professionals/be-prepared. Accessed October 25, 2018.
To comment on this article, contact rdavidson@uspharmacist.com.






