US Pharm. 2024;49(1):HS2-HS6.
ABSTRACT: Aneurysmal subarachnoid hemorrhage makes up a minority of strokes annually; however, the mortality rate is relatively high at 10% to 25%. Complications contributing to morbidity and mortality include cerebral edema, hydrocephalus, elevated intracranial pressure, and cerebral vasospasm, which can lead to delayed cerebral ischemia (DCI). Initial management should include the treatment of hypertension with short-acting agents to prevent rebleeding. Hypotension should also be avoided to not compromise cerebral perfusion and increase risk of cerebral ischemia. Additional initial considerations include targeting euvolemia and reversal of coagulopathy, if present. Patients are routinely monitored for the development of vasospasm, and nimodipine is administered for the first 21 days, as it may improve functional outcomes. If cerebral vasospasm is detected, efforts should be made to avoid the development of DCI, including targeting euvolemia, consideration of blood pressure augmentation, and intra-arterial vasodilators or cerebral angioplasty. Areas of ongoing research in DCI include the use of intraventricular calcium channel blockers and IV milrinone. Other supportive care includes thromboembolism prophylaxis after aneurysm occlusion, antiseizure medications, and standard glycemic goals for critically ill patients (targeting blood glucose ≤180 mg/dL).
Subarachnoid hemorrhage accounts for 5% to 10% of all strokes in the United States each year, and 50% of those without a preceding trauma are caused by the rupture of an intracranial aneurysm.1 The incidence of aneurysmal subarachnoid hemorrhages (aSAHs) in the U.S. is 11.4 per 100,000 patient-years, with mortality rates ranging from 10% to 25%. Incidence increases with age and primarily affects women aged 55 years and older and black patients. Other risk factors for the development of aSAH include hypertension, tobacco use, and family history.2
Intracranial aneurysms typically develop due to hemodynamic stress at branch points along intracranial arteries. Bleeding into the subarachnoid space occurs when the aneurysm ruptures. The hallmark presenting symptom is a headache that is sudden in onset and immediately reaches maximal intensity in an otherwise awake and alert patient.1,2 Recommended clinical grading scales for predicting mortality in aSAH include the Hunt and Hess (HH) and the World Federation of Neurosurgical Societies in combination with the radiographical findings (Fisher scale). Preferred initial imaging includes a noncontrast head CT scan followed by CT angiography (CTA) or digital subtraction angiography.2
Complications increasing morbidity and mortality in aSAH patients include cerebral edema, hydrocephalus, elevated intracranial pressure (ICP), and delayed cerebral ischemia (DCI). The cause of DCI is multifactorial from the effects of large-vessel cerebral vasospasm due to arteriolar constriction and cerebral microthrombosis, ischemia, blood-brain barrier breakdown, cerebral autoregulation impairment, and capillary transit time heterogeneity.2 Vasospasm is thought to occur when blood in the subarachnoid space begins to break down and release products that are irritants to the vessels. This triggers the inflammatory cascade and results in the release of macrophages and neutrophils, which in turn causes inflammatory-induced vasoconstriction.3 Vasospasm occurs in up to 70% of patients and starts 3 to 4 days after rupture, peaking at 7 to 10 days, and typically resolves by 14 to 21 days.1 Symptoms may include hemiparesis, apraxia, aphasia, neglect, hemianopia, or a decrease of Glasgow Coma Scale by two points or more.4
aSAHs are a considerable financial burden on the healthcare system, with hospital costs up to $500,000, not including posthospitalization rehabilitation and long-term care. Early repair, preferably within 24 hours of onset, of ruptured aneurysm by endovascular coiling or neurosurgical clipping is recommended to prevent rebleeding and reduce fatality. The process of coiling includes advancing a microcatheter into the aneurysm sac and depositing metal coils into the aneurysm lumen. This stops intra-aneurysmal blood flow and induces thrombus formation. If clipping is chosen, a titanium clip is placed across the neck of the aneurysm to mechanically close the sac at its neck while preserving blood flow through the adjacent normal arteries.1 Coiling is typically the preferred strategy; however, clipping should be considered in patients who are aged younger than 40 years due to durability benefits.2
Two aSAH guidelines were published in 2023, including one from the American Heart Association (AHA) in collaboration with the American Stroke Association (ASA) and another from the Neurocritical Care Society (NCS), providing comprehensive recommendations for management.2,5 The similarities and differences between the two guidelines are discussed below.
INITIAL HEMODYNAMIC MANAGEMENT
Blood Pressure Targets
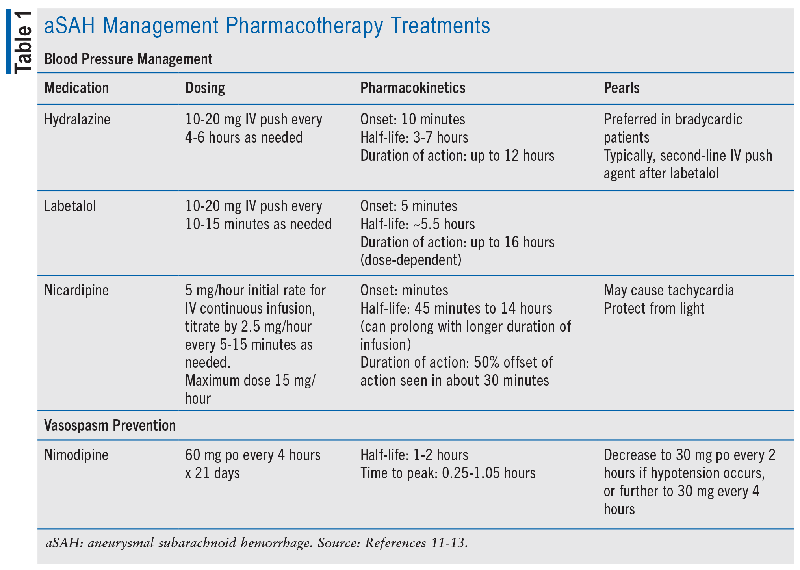
In patients presenting with aSAH, initial hemodynamic management is key to prevent rebleeding and maintain cerebral perfusion. Systolic blood pressure (SBP) above 160 mmHg has been associated with aneurysm rebleeding, but studies evaluating the treatment of elevated blood pressure (BP) are limited to retrospective and observational data and have examined variable BP targets.2,5 Both the AHA/ASA and NCS guidelines agree that there is insufficient evidence for a precise BP goal but that avoiding hypertension may minimize rebleeding.2,5 Conversely, hypotension (mean arterial pressure <65 mmHg) in combination with elevated intracranial pressure may compromise cerebral perfusion. Therefore, the AHA/ASA guidelines emphasize avoiding BP variability through frequent monitoring and control of hypertension with short-acting medications.2 Potential options for short-acting antihypertensives are listed in TABLE 1.
Fluids and Electrolytes
Volume status assessment is foundational to maintaining hemodynamic goals, and goal-directed treatment to target euvolemia is reasonable.2,5 Volume assessment methods among critically ill patients are debated, and it is thought that central venous pressure alone may not provide an adequate picture of volume status. Goal-directed therapy aided by continuous monitoring of cardiac output and stroke volume may benefit incidence of vasospasm, delayed cerebral ischemia, and functional outcome based on the findings of one small randomized, controlled trial.2
Hyponatremia has been observed in about one-half of patients with aSAH, with or without natriuresis or polyuria.2 It is not clear whether hyponatremia affects vasospasm and functional outcomes, but hyponatremia has been consistently associated with prolonged intensive care unit and hospital length of stay. Treatment of hyponatremia and polyuria with fludrocortisone has been shown to reduce natriuresis and improve sodium and volume status, but impact on delayed cerebral ischemia and functional status outcomes has not been demonstrated. Hydrocortisone in these patients may be associated with additional morbidity, but data may have been confounded by outdated practices including delayed aneurysm treatment and hypervolemic hypertensive hemodilution therapy (HHT).2 While the AHA/ASA guidelines state that it is reasonable to use mineralocorticoids to treat hyponatremia or natriuresis, the NCS guidelines state that there is insufficient evidence to support their use, as there is no proven patient-centered outcomes benefit.2,5
Coagulopathies
Early administration of tranexamic acid in patients with aSAH has not been shown to improve functional outcomes and is not recommended.2,5 However, anticoagulation reversal should be performed in patients receiving anticoagulation based on data across etiologies of intracranial hemorrhage, not aSAH specifically.2
CEREBRAL VASOSPASM AND DCI MANAGEMENT
Nimodipine is a lipid-soluble calcium channel blocker, and it is the only FDA-approved agent for the treatment of aSAH and is specifically included in the management of patients to prevent vasospasm. It crosses the blood-brain barrier and selectively inhibits smooth muscle contraction of the cerebral arteries by blocking extracellular calcium from entering the cell. Nimodipine is dosed orally 60 mg every 4 hours for 21 days, and if patients are unable to tolerate it due to hemodynamic changes, the dosage can be reduced to 30 mg every 2 hours. The benefit was published in 1983 by Allen et al, who found in a prospective, double-blind, randomized, placebo-controlled trial that eight out of 60 patients in the placebo arm died or had severe disabling deficits, while only one out of 56 in the nimodipine arm had a severe outcome (P = .03).6
Patients are typically monitored for the development of vasospasm daily from Day 4 to Day 14. The AHA/ASA 2023 guidelines recommend CTA, CT perfusion, continuous electroencephalography (cEEG), or transcranial Doppler (TCD) as reasonable options.2 TCD is commonly performed because it can be completed at bedside, is noninvasive, and does not require any contrast. To perform, a technician places a probe to the acoustic windows, regions around the skull where ultrasound waves can reach the cerebral circulation. Flow velocities of the cerebral arteries are recorded. The severity is then determined based on the change in the vessels’ speed, volume, and diameter and is classified as mild, moderate, or severe.4
Once vasospasm is detected, there are a few management options to prevent the development of DCI. These include maintaining euvolemia, which is supported by both guidelines, BP augmentation, intra-arterial vasodilators, and cerebral angioplasty.2,5
HHT is no longer recommended as the mainstay of therapy since hypervolemia has been shown to increase medical complications such as pulmonary edema, myocardial infarction, pneumonia, hyponatremia, and nosocomial infections without improving outcomes or preventing DCI.7 Therefore, euvolemia with crystalloid solutions is preferred.2 In addition, it is recommended to avoid hemodilution due to no improvement in outcomes and a worsening in adverse events.7 The last component in HHT is hypertension, which is the only remaining component of HHT potentially showing benefit. The AHA/ASA guidelines recommend BP augmentation with IV colloids or vasopressors. Vasopressor options include norepinephrine or phenylephrine and can be used to reduce the progression and severity of DCI. Further high-quality studies are needed to determine which agent is preferred, as well as BP goals. BP augmentation should not be used as prophylaxis, as this has been shown to cause harm, such as congestive heart failure. Finally, hypotension should be avoided, as this can lead to DCI.2 In contrast, the NCS guidelines that state there is insufficient evidence to recommend for or against BP augmentation. They also recommend avoiding SBP greater than 160 mmHg as this has been shown to cause rebleeding. However, caution is advised, as too low a BP can decrease cerebral perfusion and cause cerebral ischemia.5
Endovascular Treatment Options
IA vasodilators can reverse vasospasm and reduce the progression and severity of DCI. Further studies are needed to determine which agents and dosages are preferred, but calcium channel blockers (verapamil, nicardipine, and nimodipine) have been used.7 Papaverine was historically used as the first- line IA vasodilator but has fallen out of favor due to the risk of neurotoxicity.2 A second endovascular option is cerebral angioplasty. Cerebral angioplasty via transluminal balloon angioplasty is a mechanical option using endovascular balloons to mechanically dilate the vessel. This results in improved vasospasm and clinical outcomes due to longer vasodilatory effects with low complication rates.2
Additional Treatment Options
Additional vasospasm treatment options include intraventricular (IVT) calcium channel blockers and IV milrinone; however, currently there is a lack of evidence to support these treatment modalities. While the AHA/ASA guidelines do not mention IVT calcium channel blockers, the NCS guidelines state that there is insufficient quality evidence to recommend for or against it.2,5 Milrinone is an inotropic agent with vasodilatory properties. It works by inhibiting phosphodiesterase-3, which hydrolyzes cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP). An increase in cAMP leads to an increase in inotropy, and an increase in cGMP leads to arterial and venous smooth muscle relaxation. This results in increased cardiac output and vasodilation.8 With limited studies, this has been shown to prevent DCI. The AHA/ASA guidelines state that milrinone may have a beneficial effect in preventing vasospasm or DCI, while the NCS guidelines state that there is inconclusive evidence.2,5 Further studies are needed to determine the beneficial effect of IVT calcium channel blockers and milrinone.
Options recommended to avoid in the AHA/ASA guidelines as well as the NCS guidelines include endothelin antagonists, statins, and magnesium.2,5
ADDITIONAL SUPPORTIVE CARE CONSIDERATIONS
Thromboembolism Prophylaxis
After securing the ruptured aneurysm, pharmacologic or mechanical venous thromboembolism (VTE) prophylaxis is strongly recommended.2 Incidence of VTE among patients with acute aSAH has been estimated at 4% to 24%, but the exact benefit of pharmacologic VTE prophylaxis is unclear and is extrapolated from data outside aSAH. However, safety has been demonstrated with a small randomized, controlled trial, and retrospective cohort studies demonstrating no significant bleeding risk with the use of low-molecular-weight heparin after the aneurysm is secured. Optimal timing of VTE prophylaxis has not been fully explored. Data are limited to a case-control study that demonstrated no difference in intracranial hemorrhage when initiating VTE prophylaxis ≤24 hours or >24 hours after aneurysm occlusion.2
Seizure Prophylaxis
Seizure prophylaxis is not routinely recommended in aSAH. However, in those considered high risk, seizure prophylaxis may be considered, and cEEG monitoring may aid in detecting seizure. High-risk patients include those with high-grade aSAH (i.e., HH grade ≥3), hydrocephalus, intraparenchymal hemorrhage, ruptured middle cerebral artery aneurysm, or cortical infarction.2 For new-onset seizures or high-risk prophylaxis, there are no formal recommendations on which antiepileptic medication should be used. However, phenytoin should be avoided, as it has been associated with increased morbidity and mortality.2 Recommended treatment duration for seizures on initial presentation is 7 days.2 However, early-onset seizures (≤7 days) and late seizures (>7 days) may be related to nonhemorrhage etiologies such as infarction and warrant expert consultation for diagnostic workup and optimal treatment duration.
Glycemic Control
Per guidelines, effective glycemic control with strict hyperglycemia management and avoidance of hypoglycemia are reasonable.2 While hyperglycemia on presentation or within 72 hours of presentation has been associated with vasospasm, delayed cerebral ischemia, and unfavorable functional outcomes, there is no evidence to suggest a unique glycemic management for patients with aSAH.2 Therefore, a blood glucose of ≤180 mg/dL should be targeted, as this has been associated with reduced mortality in critically ill patients and is the standard of care.9
CONCLUSION
Pharmacists play an integral role in the care of patients presenting with aSAH. Complications associated with aSAH include cerebral edema, hydrocephalus, elevated ICP, and DCI. Nimodipine is the only current therapy approved to help reduce the incidence and severity of vasospasm leading to DCI. Current Joint Commission National Quality Measures recommend administering nimodipine within 24 hours of arrival to the hospital, and pharmacists can help ensure appropriate medication timing and administration.10 Additional vital management includes BP regulation, fluids and electrolytes, seizure prophylaxis, thromboprophylaxis, and glucose control. In addition to recently published guidelines, pharmacists can utilize their expertise to help improve outcomes for these critically ill patients. Further research is needed to solidify recommendations regarding ideal BP targets, antihypertensives, and antiepileptics in aSAH and treatment strategies for vasospasm management.
REFERENCES
1. Lawton MT, Vates GE. Subarachnoid hemorrhage. N Engl J Med. 2017;377(3):257-266.
2. Hoh BL, Ko NU, Amin-Hanjani S, et al. 2023 Guideline for the management of patients with aneurysmal subarachnoid hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke. 2023;54(7):E314-E370.
3. Carr KR, Zuckerman SL, Mocco J. Inflammation, cerebral vasospasm, and evolving theories of delayed cerebral ischemia. Neurol Res Int. 2013;2013:506584.
4. Samagh N, Bhagat H, Jangra K. Monitoring cerebral vasospasm: how much can we rely on transcranial Doppler. J Anaesthesiol Clin Pharmacol. 2019;35(1):12-18.
5. Treggiari MM, Rabinstein AA, Busl KM, et al. Guidelines for the neurocritical care management of aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2023;39(1):1-28.
6. Allen GS, Ahn HS, Preziosi TJ, et al. Cerebral arterial spasm—a controlled trial of nimodipine in patients with subarachnoid hemorrhage. N Engl J Med. 1983;308(11):619-624.
7. Li K, Barras CD, Chandra RV, et al. A review of the management of cerebral vasospasm after aneurysmal subarachnoid hemorrhage. World Neurosurg. 2019;126:513-527.
8. Lannes M, Teitelbaum J, del Pilar Cortés M, et al. Milrinone and homeostasis to treat cerebral vasospasm associated with subarachnoid hemorrhage: the Montreal Neurological Hospital protocol. Neurocrit Care. 2012;16(3):354-362.
9. NICE-SUGAR Study Investigators. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283-1297.
10. The Joint Commission. Specifications manual for Joint Commission National Quality Measures: measure information form. https://manual.jointcommission.org/releases/TJC2024A1/MIF0293.html. Accessed December 12, 2023.
11. Hydralazine: drug information. In: Lexicomp Online. Waltham, MA: UpToDate, Inc; 2023. https://online.lexi.com. Accessed October 15, 2023.
12. Labetolol: drug information. In: Lexicomp Online. Waltham, MA: UpToDate, Inc; 2023. https://online.lexi.com. Accessed October 15, 2023.
13. Nicardipine: drug information. In: Lexicomp Online. Waltham, MA: UpToDate, Inc; 2023. https://online.lexi.com. Accessed October 15, 2023.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.






