US Pharm. 2010;35(1):30-32.
Carpal tunnel syndrome (CTS) is a condition whereby increased pressure in the carpal tunnel exists, affecting median nerve function in the wrist.1 The pressure ultimately affects microvascular circulation and is increased by movement of the wrist and flexion of the fingers, leading to hand discomfort and functional impairment. It has been estimated that 4 to 10 million Americans are afflicted with CTS; however, it is difficult to determine an exact number due to the fact that there is no standard definition or gold standard for diagnosis of the condition.2 CTS is prevalent in approximately 3 to 6 million women and 1 to 4 million men.2 In 2005, CTS was found to be a leading cause of work-related disability, resulting in a median of 27 days away from work for employees and a significant cost to employers.3 The medical costs of this condition in the U.S. have been estimated to exceed $2 billion annually.3
Risk Factors
Several studies have indicated that the repetition, vibration, and forceful physical demands on the wrist that are required in such jobs as construction and manufacturing lead to the development of CTS.3,4 Contrary to what is typically believed, studies show that jobs requiring typing and computer use present little risk of developing CTS.3 Other diseases or conditions that may contribute to CTS include infections such as Lyme disease; inflammatory conditions such as gout and rheumatoid arthritis; diabetes; obesity; and pregnancy.5
Symptoms
CTS begins gradually, with an initial presentation of periods of numbness and discomfort in the hands. This is a direct result of pressure on the median nerve, which leads to interruption of conduction within the large myelinated nerve fibers. As the condition progresses, pain and muscle atrophy can ensue due to ischemic changes within the nerve fibers. Symptoms can range from mild numbness to major functional impairment depending on the extent of nerve involvement (TABLE 1).6,7
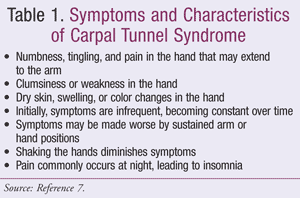
Diagnosis
Although there is no gold standard for the diagnosis of CTS, nerve conduction studies are often used.5,8 The study results are not absolute for diagnosis, as false-positive and false-negative results often occur. Clinical tests used to aid in diagnosis include Phalen’s maneuver and Tinel’s sign.8 Phalen’s maneuver consists of flexing the wrist to a 90-degree angle for 1 minute. The test is considered positive if pain or numbness occurs during this time frame. Tinel’s sign involves tapping over the carpal tunnel and is considered positive if paresthesia occurs in the fingers. Vibration and monofilament testing can also be used to determine sensation in the carpal tunnel.5
Nonpharmacologic Treatment
Nonpharmacologic treatment of CTS includes splinting of the wrist at a neutral angle.5 The splint should be used within 3 months of the onset of symptoms.5 When it is worn properly, 80% of patients report improvement in their condition within days.8 The splint should be worn particularly at night and also throughout the day depending on the patient’s lifestyle and activities.5 Nonpharmacologic and nonsurgical treatment is preferred in pregnancy, making splinting a viable choice.6 Ultrasound therapy has also been used in the treatment of CTS; however, efficacy is questionable.5,9
Medications
Medications used for the treatment of CTS include nonsteroidal anti-inflammatory drugs (NSAIDs), diuretics, pyridoxine (vitamin B6), and corticosteroids.
Nonsteroidal Anti-Inflammatory Drugs: NSAIDs, diuretics, and oral steroids are often used to treat symptoms of CTS. A randomized, double-blind, placebo-controlled study was conducted in 73 patients with CTS for a duration of 4 weeks.10 Patients were randomized to one of four groups: 4 weeks of placebo, 4 weeks of tenoxicam-SR 20 mg daily (a prescription-only NSAID available in the United Kingdom), 4 weeks of trichlormethiazide 2 mg daily (a diuretic), or 2 weeks of prednisolone 20 mg daily followed by 2 weeks of prednisolone 10 mg daily. Patients were assessed based on a global symptom score (GSS) that included five areas: pain, numbness, paresthesia, weakness/clumsiness, and nocturnal awakening. Scores ranged from 0 to 50, with zero indicating no symptoms and 50 indicating severe symptoms. At 2 weeks, patients in the prednisolone group had a statistically significant reduction in GSS (27.9 vs. 15.0 from baseline to 2 weeks, P = .0002) compared to patients in the other groups. The same statistical significance was held at 4 weeks for patients in the steroid group (15.0 vs. 10.0 from 2 weeks to 4 weeks, P = .0001). Based on this information, it has been determined that a short course of treatment with oral steroids appears to be superior to treatment with diuretics and NSAIDS; however, symptoms generally reappear, and many patients do not experience relief without surgery.10
Since there are no studies evaluating NSAIDs alone in patients with CTS, conclusions about efficacy can be drawn from this study. Many physicians initiate an NSAID to alleviate symptoms; however, these drugs do not provide relief for many patients. It may be reasonable to give patients with mild-to-moderate symptoms of CTS a trial of an NSAID, keeping in mind that improvement does not usually occur before 2 weeks and may be short-lived, and that surgery may be imminent.10
Diuretics: Diuretics are often prescribed for the treatment of CTS based on the principle that an increase in fluid in the carpal tunnel leads to an increase in pressure, thereby causing symptoms.11 A double-blind, controlled trial was conducted on 81 symptomatic hands of patients diagnosed with CTS by nerve conduction testing.11 Patients were randomized to receive bendrofluazide 5 mg daily for 4 weeks or placebo. Improvement in symptoms was gauged by a numerical score ranging from 0 (no improvement) to 5 (full recovery). The results of the study were not statistically significant; however, 46% of hands in the treatment group experienced improvement compared to 50% in the placebo group. Nerve conduction studies were performed at 6 months on the hands of those patients who experienced a clinical improvement in the study. Patients in the treatment group experienced statistically significant improvement in motor and sensory latency while those in the placebo group experienced statistically significant improvement only in motor latency. Thus, diuretics are an option for initial treatment; however, available study results show little, if any, improvement in symptoms.
Pyridoxine: It has been proposed that CTS may result from a deficiency of pyridoxine. This is based on the principle that peripheral neuropathy, also a condition affecting the nerves, can result from a lack of vitamin B6.12 As a result, researchers have assessed the benefit of using pyridoxine as a treatment for CTS. A randomized, double-blind, placebo-controlled trial was conducted in 32 patients with CTS.12 Patients were treated for 12 weeks with 200 mg per day of pyridoxine or placebo. Symptom severity was measured by using a 5-point scale (0 = no pain, 5 = a great deal of pain). Over the course of the study, patients in the treatment group experienced a statistically significant reduction in swollen fingers (mean change in pain scores -0.8 [treatment] vs. -0.3 [placebo], P <.05) and tingling and discomfort in the hands after repetitive movements (mean change in pain scores -1.4 [treatment] vs. -0.4 [placebo], P <.001) as compared to patients in the placebo group, who experienced no significant change. However, the occurrence of such symptoms as nocturnal pain, numbness, and tingling was not changed significantly by treatment with pyridoxine. Since nocturnal pain is the symptom that is most bothersome to patients, the investigators concluded that the use of pyridoxine is not beneficial, especially since there is a risk of toxicity with its use.12
Corticosteroids: Oral and injectable corticosteroids are used for the treatment of CTS in patients who continue to experience mild-to-moderate symptoms after lifestyle modifications and splinting.8 Oral steroids have been used and have been found to be more effective than NSAIDs, diuretics, or pyridoxine10; however, they are not as effective as a local steroid injection.8 A prospective, randomized, double-blind, parallel treatment study was conducted in 60 patients who received either oral placebo daily for 10 days in conjunction with a single 15 mg methylprednisolone acetate injection into the carpal tunnel or 25 mg daily of prednisolone for 10 days and a single saline injection into the carpal tunnel.13 Mean GSS score was used to assess improvement with possible scores ranging from 0 (absence of symptoms) to 50 (most severe symptoms). Mean GSS scores between the groups was not statistically significant at 2 weeks. At 8 weeks and 12 weeks, the steroid injection group obtained statistical significance, P = .002 and P = .004, respectively. Mean GSS scores for the study are shown in TABLE 2. Side effects were minimal in both study groups and included injection site pain, insomnia, bloating, and polyphagia. The study implies that steroid injections are superior to oral steroids both in efficacy and duration as evidenced by a decline in mean GSS scores in patients in the injection group.13
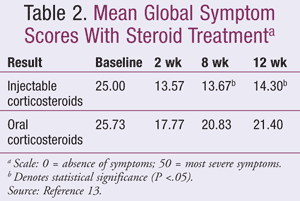
The initial response rate to a steroid injection is about 70%; however, relapse is common and generally occurs within 1 to 2 years after administration.1,8 Some patients experience a recurrence in as little as a few months after the injection.14 Steroids are combined with a local anesthetic such as lidocaine and injected at either of two locations, directly into the carpal tunnel or proximal to the carpal tunnel.5 The latter technique lowers the risk of damaging the median nerve and can help to alleviate swelling around the area often associated with CTS. As with steroid injections for any condition, the number given per year should be limited to avoid injury to the tendons and nerves.8
Combination Therapy
In theory, it appears that a combination of the drugs discussed previously may be beneficial to the patient; however, no studies have been conducted to verify this. In addition, the treatment guidelines for CTS developed by the American Academy of Orthopaedic Surgeons do not address or recommend combination pharmacologic therapy.15
Surgery
For most patients with continued CTS pain despite treatment with splinting, oral medications, and steroid injections, surgery is the only option for relief.5 Carpal tunnel decompression is performed on an outpatient basis and provides a complete cure for most patients. The surgery is performed by open or endoscopic procedures, and it is debated as to which procedure is most effective. It is possible that the patient may not experience relief from the procedure; however, this is typically due to a misdiagnosis of the condition, failure to fully divide the transverse carpal tunnel ligament during surgery, or failure to perform surgery before the median nerve is permanently damaged.1
Alternative Therapies
Although alternative therapies are not studied comprehensively, they are used by many patients, making knowledge of them imperative for pharmacists. Topical safflower plant extract ointment (Zolacet) is marketed and available on the Internet for patients to purchase for the relief of CTS pain.16 It has been proposed that safflower plant extract exhibits anti-inflammatory and immunomodulating properties, which may make it beneficial in CTS. In addition, it has several flavonoids that may have analgesic effects. A small, open-label study was conducted in 66 patients with chronic pain, 12 of whom had CTS confirmed by electrodiagnostic testing.17 In most cases, CTS patients failed to respond to NSAIDs, and 17% failed to respond to steroid injections. Patients were treated for 4 weeks with a topical, concentrated safflower plant extract ointment. Patients were instructed to apply the ointment three to four times daily. Of the 12 patients with CTS, 82% experienced significant relief, with some reporting complete relief by the end of the 4-week period. When compared to patients with back, neck, and tendon pain, patients with CTS achieved a statistically significant improvement in pain scores (P = .0004, P = .039, P = .22, respectively). Information on the product is limited; however, the only side effect reported in the study was a mild rash.
Conclusion
The risk factors for developing CTS are often debated; however, the condition is very painful for those patients afflicted by it. Because it is one of the major conditions that affect workers today, pharmacists are likely to encounter patients with CTS. Based on the studies available, it appears that NSAIDS, diuretics, and oral steroids may work for a subset of patients with mild-to-moderate symptoms. Injectable corticosteroids appear to have the most beneficial outcomes in improving patient symptoms associated with CTS; however, the effects are often short-lived and rarely curative. Based on the limited studies with pyridoxine and the potential for toxicity, it is best to avoid this therapy in patients. In patients who fail to respond to oral therapy or wrist splinting, surgery is likely to result in a cure of the condition; however, surgery is not without risks and at times may fail to produce the desired outcome of a cure.
REFERENCES
1. Bland JD. Carpal tunnel syndrome. BMJ. 2007;335:343-346.
2. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35.
3. Armstrong T, Dale AM, Franzblau A, Evanoff BA. Risk factors for carpal tunnel syndrome and median neuropathy in a working population. J Occup Environ Med. 2008;50:1355-1364.
4. Kao SY. Carpal tunnel syndrome as an occupational disease. J Am Board Fam Pract. 2003;16:533-542.
5. Viera AJ. Management of carpal tunnel syndrome. Am Fam Physician. 2003;68:265-272.
6. Whitley JM, McDonnell DE. Carpal tunnel syndrome. A guide to prompt intervention. Postgrad Med. 1995;97:89-96.
7. American Academy of Orthopaedic Surgeons. Carpal tunnel syndrome.
http://orthoinfo.aaos.org/
8. Katz JN, Simmons BP. Clinical practice. Carpal tunnel syndrome. N Engl J Med. 2002;346:1807-1812.
9. Gerritsen AA, de Krom M, Struijs MA, et al. Conservative treatment options for carpal tunnel syndrome: a systematic review of randomised controlled trials. J Neurol. 2002;249:272-280.
10. Chang MH, Chiang HT, Lee SS, et al. Oral drug of choice in carpal tunnel syndrome. Neurology. 1998;51:390-393.
11. Pal B, Mangion P, Hossain MA, Wallace AS. Should diuretics be prescribed for idiopathic carpal tunnel syndrome? Results of a controlled trial. Clin Rehabil. 1988;2:299-301.
12. Spooner GR, Desai HB, Angel JF, et al. Using pyridoxine to treat carpal tunnel syndrome. Can Fam Physician. 1993;39:2122-2127.
13. Wong SM, Hui AC, Tang A, et al. Local vs systemic corticosteroids in the treatment of carpal tunnel syndrome. Neurology. 2001;56:1565-1567.
14. Lee JH, An JH, Lee SH, Hwang EY. Effectiveness of steroid injection in treating patients with moderate and severe degree of carpal tunnel syndrome measured by clinical and electrodiagnostic assessment. Clin J Pain. 2009;25:111-115.
15. Guideline on the treatment of carpal tunnel syndrome. American Academy of Orthopaedic Surgeons.
www.aaos.org/Research/
16. Zolacet Daily Pain Defense Cream.
www.4sale4now.com/products/
17. Hanania M, Duarte R, Livingstone D, et al. Topical safflower plant extract for chronic pain: a prospective, open-label study. Integr Med. 2005;4:16-20.
To comment on this article, contact rdavidson@uspharmacist.com.





