US Pharm. 2009;34(9)(Oncology suppl):3-11.
ABSTRACT: Intravenous (IV) chemotherapeutic medications have the potential to cause extravasation injury or tissue necrosis at the site of administration. Although a majority of extravasations can be prevented by proper and thorough infusion administration, several antidotes have been recognized and studied to prevent further tissue injury.
Infiltration and extravasation are two well-known, distinct complications of IV infusion therapy. According to the Infusion Nurses Society and the Oncology Nursing Society, both complications involve the inadvertent leakage of an IV solution into surrounding tissue; however, the type of solution differs. Infiltration is the inadvertent administration of a nonvesicant or irritant solution or medication into the surrounding tissues.1,2 Nonvesicants are agents that rarely produce acute reactions or destroy the tissue when they infiltrate. Irritant agents can induce pain at the injection site or along the vein, with or without an inflammatory reaction, usually with no persistent tissue damage. However, irritants may cause soft tissue ulcers only if a large amount of concentrated drug solution is inadvertently extravasated to induce inflammatory reactions with no persistent tissue damage.3,4
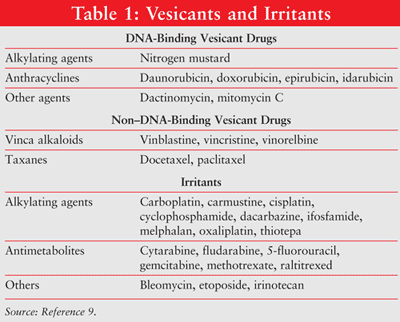
Extravasation refers to the inadvertent instillation or leakage of vesicant material into the perivascular and subcutaneous spaces during medication administration.1,2 Extravasation results in local reactions ranging from local irritation to severe tissue necrosis of the skin, surrounding vasculature, and supporting structures.5-8 Chemotherapeutic agents may be classified, based on their potential to cause local tissue injury, as vesicants, irritants, or nonvesicants. (Several vesicants and irritants are outlined in TABLE 1.9) The accidental extravasation of IV drugs occurs in approximately 0.1% to 6% of peripheral IV infusions and 0.3% to 4.7% of implanted venous access port infusions.10-12
Pathophysiology of Tissue Damage
The extent of tissue damage depends on the chemotherapeutic agent’s binding capacity to DNA. DNA-binding agents include anthracyclines, antitumor antibiotics, platinum analogues, and some alkylating agents.3,4 DNA-binding antineoplastics cause tissue damage by propagating lethal DNA crosslinking or strand breaks caused by free radicals, which lead to cell apoptosis.13-17 Non–DNA-binding antineoplastics (e.g., vinca alkaloids, taxanes, topoisomerase inhibitors) interfere with mitosis. Non–DNA-binding agents are cleared more easily from extravasation sites and cause less tissue damage than DNA–binding-agents.17
Risk Factors for Extravasation
Risks for extravasation are multifactorial and are largely modifiable. Many of these risks stem from errors in device use and placement. IV device factors that affect extravasation risk include needle material (i.e., metal), cannula size (large plastic vs. small plastic), and catheter type (i.e., central venous catheter [CVC]). Metal needles may cause more damage on insertion and are inflexible within the vessel, while large-gauge needles may impede blood flow, slowing the dilution of infusate.18,19 Extravasation from CVCs can occur with needle displacement from an implanted venous access port (IVAP), mechanical occlusion and subsequent CVC damage, catheter migration, or fibrin sleeve formation and thrombosis. Incorrect placement of IVAP is more likely to occur in patients with new devices, those with significant postoperative swelling, and obese patients or large-breasted women.20 Mechanical occlusions may be due to thrombus formation, drug precipitate, retrograde catheter displacement, or pinch-off (i.e., an intermittent and positional catheter occlusion; it should be suspected if infusion occlusion can be relieved by having the patient move their arm positioning).2,21,22 Avoidance of extravasation highly depends on proper maintenance of the IV site and ensuring that the correct rate of infusion is being implemented. These risk factors are related to the antineoplastics used, the patients, and the clinician administering the drug.
Prevention
Close monitoring of patients and the education of both patients and practitioners about the risks and management of vesicant extravasation seem to be the cornerstones of prevention. While physicians bear the responsibility of writing accurate orders, nurses are responsible for ensuring the skilled and proper placement of devices and monitoring of chemotherapy infusions visually and through patient interviews. During vesicant administration, the site should be monitored for swelling, redness, and pain. Vesicants should be administered in accordance with manufacturers’ recommendations (e.g., proper dilution, specified administration time). IV lines should be flushed before and after chemotherapy administration. The pharmacist’s role would be to help develop policies that will ensure the proper verification, labeling, and dispensing of chemotherapeutic agents. One way this could be executed is to use discriminative labeling when dispensing vesicant antineoplastics so that nurses use the appropriate precautionary measures. Pharmacists may play an integral role in education by providing drug information and routine in-services to all members of the health care team on the proper use and administration of the various chemotherapeutic agents. A multidisciplinary collaborative approach must be taken in order to ensure that safe practices are implemented. Administration of chemotherapy in patients requires the proper training of staff and education of patients and administrative support through the implementation of policy.2,23
Documentation of Events
Each incident of extravasation must be thoroughly documented and reported. Documentation serves several purposes, including providing an accurate account of what happened, protecting the health care professionals involved, gathering information on extravasations, and highlighting deficits in practice.23 Documentation and reporting to the appropriate entities of adverse drug reactions and medication errors is another way pharmacists can help prevent future events. Data gathered from the reported adverse drug reactions and medication errors could be used as learning tools to examine systemically the means by which these events occur. Local procedures and protocols are paramount to the timely recognition and management of extravasation and the prevention of serious tissue damage. Collaborative efforts should be made to ensure timely multidisciplinary attempts to educate all members of the health care team involved in the management of these patients once the event has occurred. Additional efforts should be made to initiate policies that would prevent further events. If policies are in place, they should be made readily available and updated regularly to reflect current practices. The European Oncology Nursing Society (EONS) 2007 extravasation guidelines provide suggested data and templates that may be used to collect information in the event of a case.23
Signs and Symptoms of Extravasation
Signs and symptoms of vesicant extravasation include swelling, redness, and/or discomfort that are often described as burning or stinging. Resistance during drug administration, a slow or sluggish infusion, and lack or loss of blood return from the IV cannula, implanted port, or other central venous access device may be indicators that a vesicant extravasation is occurring.23 The discoloration and skin indurations may progress further with the developments of blisters or necrosis and possibly ulceration and deep tissue injury. It may take several days for the full extent of the epithelial damage to be realized.
Treatment of Chemotherapy-Induced Extravasation
Treatment modalities utilized for the management of vesicant extravasation include immediate discontinuation of chemotherapy and cooling or dilution of the site of extravasation. To reduce the morbidity associated with extravasation, it is vital that clinicians are well informed of the treatments available and work quickly to avoid further patient harm. Management of nonvesicant extravasation typically includes elevating the limb and cooling and does not usually include the use of pharmacologic therapy.23
Initial Management of Vesicant Extravasation
Ease in providing early intervention may be facilitated by making an extravasation kit available within the institution. Most extravasation kits contain disposable syringes and cannulas, cold-hot packs, gauze pads, adhesive plaster, sterile and protective gloves, and medications to treat extravasation (e.g., hyaluronidase, dimethyl sulfoxide [DMSO] 99%, dexrazoxane). A list of the kit’s contents should be placed within the kit and regular verification of the drug contents with updated expiration dates should be maintained regularly by a pharmacist.2
Once vesicant extravasation occurs, the clinician should immediately stop the infusion; however, the cannula or noncoring port needle should be kept in place and an attempt to aspirate the vesicant with a 10-mL syringe should be made. The extravasation area should be marked and photographed; then the cannula or needle should be removed and the physician should be notified. Patients may require the use of analgesics to help manage the pain they may experience. Once initial measures have been taken, it is very important to thoroughly document the adverse drug reaction and/or medication error that has occurred.23
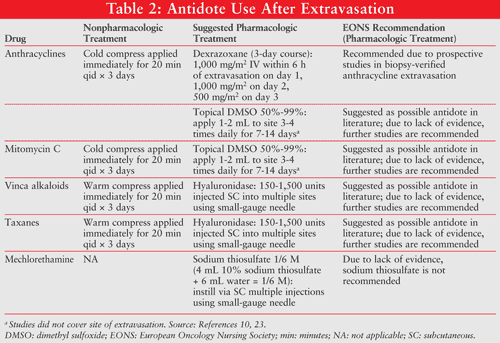
For vinca alkaloids, where dispersal or dilution of the vesicant is indicated, apply local heat for 20 minutes 4 times daily for 1 for 2 days. For other vesicant extravasations, including anthracyclines, initial treatment is geared toward localizing and neutralizing the extravasated agent with cold compresses for 20 minutes 4 times daily for 1 to 2 days, limiting the cellular uptake of these agents.23
Pharmacologic Treatment
DMSO: DMSO is a common solvent that penetrates tissues and enhances skin permeability, which may facilitate absorption of an extravasated drug. When applied topically, it has free-radical scavenging/anti oxidant properties that may speed up the removal of the extravasated drug. DMSO (Rimso-50) is available only for retail purchase in the United States in a 50% (vol/vol) solution in water. This solution is FDA approved only for the treatment of recurrent interstitial cystitis.24 The efficacy of DMSO for the treatment of chemotherapy extravasation was first described in several case reports and small clinical studies.
In one case, a 10-mg daunorubicin extravasation in a 42-year-old man was treated with sodium bicarbonate, dexamethasone (4 mg), ice packs, and 1.5 mL of topical DMSO 70%, which was applied every 3 to 4 hours for 10 days. This regimen provided pain relief and ulcer prevention.25
Olver and Schwarz reported three cases of extravasation resolution with DMSO use.26 In one case, the daily application of DMSO 99% for 14 days resulted in complete resolution of pain within 2 days with no ulceration in a patient with doxorubicin extravasation. The patient also received sodium bicarbonate 8.4% and the appropriate nonpharmacologic treatment. (See TABLE 2 for nonpharmacologic options.) In another case, a 49-year-old man receiving 4 to 6 mg of doxorubicin was treated for extravasation of doxorubicin with 5 mL of 8.4% sodium bicarbonate, ice packs, and DMSO 99% applied daily for 14 days. Ulceration did not occur; however, a 3 x 2.5 cm indurated area limiting elbow extension occurred.26 In a third case, a topical application of DMSO 99% (every 6 hours for 1 week then twice daily for another week) prevented ulceration; however, residual pigmentation remained.26
Berghammer et al also reported a case of docetaxel extravasation.27 The extravasate was diluted with normal (0.9%) saline solution and treated with ice packs. DMSO was applied 3 times every 45 minutes, and oral corticosteroids and diclofenac were given on the day of extravasation. All symptoms resolved within 24 hours; however, delayed symptoms (brown discoloration, skin hyperplasia) appeared on day 5 and increased thereafter. No plastic surgical intervention was necessary. Although DMSO in combination with hypothermia and isotonic saline solution was not effective in preventing the delayed symptoms, it did restrict the inflammation and tissue necrosis.
Ludwig et al reported that the use of a DMSO 90% and alpha-tocopherol 10% mixture within 48 hours after extravasations of anthracyclines or mitomycin C in 8 patients resulted in no skin ulceration or functional or neurovascular impairment.28 Unfortunately, alpha-tocopherol is not a readily available medicinal agent and further randomized trials should be conducted to validate its use.
In a prospective study of 20 patients with anthracycline extravasations, DMSO 99% was administered twice, 6 times daily for 2 weeks.29 No ulcers were described following DMSO treatment, although initial swelling (85%), erythema (75%), and pain (60%) were evident. After a 3-month follow-up period in 16 patients, 6 (38%) patients had no apparent symptoms and 10 (63%) had pigmented indurations. Bertelli also conducted a prospective study evaluating the effects of DMSO on anthracycline extravasations.30 Doxorubicin accounted for 11 cases and epirubicin accounted for 46 cases. Patients were treated with DMSO 99% every 8 hours for 7 days. Seventy percent of patients (40/57) had complete resolution of extravasation within 1 week, one patient had residual soft tissue induration, and one patient had residual hyperpigmentation.
In these case reports and small studies, extravasations were diagnosed clinically and not by biopsy. DMSO may be an option for the treatment of extravasated anthracyclines, mitomycin C, and actinomycin D. It is recommended that a thin layer of DMSO be applied to the extravasated area and allowed to dry. A nonocclusive dressing should be applied within 10 to 25 minutes. These steps should be repeated every 8 hours for 7 days.23 Although DMSO has been used in practice as an option for treatment, lack of prospective trials can limit its use. The EONS 2007 extravasation guidelines recommend that further studies be conducted; however, DMSO 50% to 99% may be considered for use at the discretion of the clinician.23 It is important to note that none of the DMSO prospective studies used a bandage in order to allow the skin to fully absorb the DMSO.
Hyaluronidase: It is thought that hyaluronidase is able to break down the extracellular matrix that underlies epithelial cells. The matrix holds on to large amounts of water molecules, and upon its breakdown, it is able to release the fluid into the extracellular milieu. In the case of an extravasation, it allows for increased fluid to the site, thus diluting the actual concentration of the skin at the site of contact.31,32 Hyaluronidase has been used for the treatment of vinca alkaloid and taxane extravasations.33 Doses of hyaluronidase ranging from 150 to 1,500 units diluted in 1 mL of normal saline subcutaneously or intradermally within 1 hour of extravasation have been used.
In a small clinical study of 6 patients with vinblastine (n = 1), vincristine (n = 1) and vinorelbine (n = 4) extravasation, 250 units of hyaluronidase were injected into the indwelling catheter, or subcutaneously (6 injections) in the case of injury. No steroids or cold packs were applied. Pain resolved within days of treatment with hyaluronidase in all patients. One patient complained of mildly painful induration 3 months after the extravasation of vincristine.30 These reports do reveal promising information; however, there is still an overall lack of evidence for the use of hyaluronidase for the treatment of vinca alkaloid extravasations. More prospective trials must be conducted. The EONS 2007 extravasation guidelines recommend that further studies be conducted; however, hyaluronidase may be considered as an option at suggested doses at the clinician’s discretion.23
Sodium Thiosulfate: Sodium thiosulfate prevents alkylation and tissue destruction by providing a substrate for alkylation in the subcutaneous tissue. Sodium thiosulfate has been demonstrated to be effective in animal experiments and in one case report for the treatment of mechlorethamine (nitrogen mustard) extravasation.33,34 The suggested dose is 2 mL of 0.17 M (a solution of 4 mL 10% sodium thiosulfate and 6 mL sterile water for injection). Due to lack of evidence, the EONS 2007 extravasation guidelines do not recommend this as an antidote.22
Dexrazoxane: Dexrazoxane (Totect) was recently approved by the FDA for the treatment of anthracycline-induced extravasations. Dexrazoxane has a dual mechanism of action: 1) it acts as an iron chelator, preventing formation of iron-anthracycline complexes and iron-mediated hydroxyl radicals that cause oxidative damage; and 2) it stabilizes topoisomerase II and makes it inaccessible to anthracycline chemotherapy. Dexrazoxane blocks the enzyme so that it is no longer affected by anthracycline and prevents damage to the healthy cells in tissue.35
Dexrazoxane efficacy was demonstrated in two clinical trials conducted in Europe.36 Both trials were prospective, open-label, single-arm, multicenter clinical trials conducted from July 2001 to August 2005, with a total of 80 patients enrolled. The first study enrolled patients from 17 cancer sites in Denmark, and the second enrolled patients from 34 cancer sites in Denmark, Germany, Italy, and the Netherlands. Anthracycline extravasation was diagnosed and confirmed via fluorescence microscopy of biopsied tissue taken within 6 hours of dexrazoxane. A sample size of 32 assessable patients was required to show a 70% or greater reduction in the incidence of surgery (one-sided binomial test, a = .025, ß = .20). The primary end point was the rate of surgical resection and necrosis. Fifty-four of the 80 patients (study 1: n = 18; study 2: n = 36) were evaluable (n = 13 negative biopsies; n = 4 no biopsies performed; n = 8 protocol violations), with a mean age of 56 years (range, 34-81 years). Sixty-nine percent (n = 37) of the patients evaluated were female. Dexrazoxane was given as an IV infusion over 1 to 2 hours; 1,000 mg/m2 within 6 hours; 1,000 mg/m2 after 24 hours; and 500 mg/m2 after 48 hours of extravasation.36
Patients were assessed for efficacy and safety at days 7, 14, 21, and 28, and for efficacy at day 90.36 The most common cancer diagnosis was breast cancer (50%), followed by lymphoma (39%) and other types of cancer (9%). Patients experienced extravasations of doxorubicin (n = 23) or epirubicin (n = 31). The mean extravasation area was 23.6 cm2 in study 1 and 39 cm2 in study 2. Eleven patients had areas of extravasation exceeding 75 cm2. There were no incidences of surgery in study 1 and 1 incident (2.8%, 95% CI 0.1%-14.5%) in study 2. The patient who developed tissue necrosis due to extravasation had a very large area of doxorubicin extravasation measuring 253 cm2. Symptoms increased in the days after the extravasation, tissue necrosis began to occur 9 days following the patient’s extravasation, and the necrosis was surgically excised. In study 1, there was 1 (5.6%) incident of necrosis due to biopsy, 6 (33.3%) incidents of postponement or cancellation of scheduled cancer treatment, and 9 (50%) hospitalizations due to the extravasation. In study 2, there was 1 (2.8%) incident of necrosis due to extravasation, 3 (8.3%) incidents of necrosis due to biopsy, 10 (27.8%) incidents of postponements or cancellation of scheduled cancer treatment due to the extravasation, and 13 (36.1%) incidents of hospitalization due to the extravasation. At 3-month follow-up, 88.9% (n = 16) had no negative sequelae in study 1 and 63.9% (n = 23) in study 2. Fifteen of the 80 patients experienced sensory disturbances (n = 9), skin atrophy (n = 5), pain (n = 10), disfigurement (n = 1), and limitation (n = 3). Most of the patients (71%) were able to receive further chemotherapy treatment on schedule.
The most common adverse effects included a decreased white blood cell count (72.5%), decreased hemoglobin (42.5%), decreased platelet count (26%), increased aspartate aminotransferase (36.8%), increased alanine aminotransferase (23.9%), injection site reaction (27.5%), and nausea (18.8%).36 Results from the clinical trials revealed that dexrazoxane is an effective agent for the treatment of acute anthracycline extravasations and prevention of delayed symptoms.
A summary of these treatment recommendations, including the EONS 2007 guidelines, is provided in TABLE 2.
Role of the Pharmacist
Pharmacists should be closely involved in the assessment and management of all chemotherapy-induced extravasations. They can play an integral role in several ways, including: 1) development of policies and protocols; 2) patient, family, and multidisciplinary education; 3) documentation and reporting of extravasations and other adverse drug reactions subsequent to therapy; and 4) monitoring the proper dosing, administration, and efficacy of treatment.
Conclusion
All of the studies reviewed involved adults, as pediatric patients would have had an increased risk of agitation and pulling of the lines. To date, dexrazoxane is the only extravasation agent that has been studied in large, multicenter, prospective trials that used objective, reliable testing to diagnose extravasation cases; this provides strong evidence of dexrazoxane’s utility. Despite their common uses in practice, other pharmacologic treatments, such as DMSO and hyaluronidase, have not been studied extensively; therefore, recommendations based on evidence are lacking. Management of extravasation should be conducted at the discretion of the clinician in collaboration with all members of the health care team.
REFERENCES
2. Polovich M, White J, Kelleher L. Chemotherapy and Biotherapy Guidelines and Recommendations for Practice. 2nd ed. Pittsburgh, PA: Oncology Nursing Society; 2005.
3. Ener RA, Meglathery SB, Styler M. Extravasation of systemic hemato-oncological therapies. Ann Oncol. 2004;15:858-862.
4. Sauerland C, Egelking C, Wickman R, Corbi D. Vesicant extravasation part I: mechanisms, pathogenesis, and nursing care to reduce risk. Oncol Nurs Forum. 2006;33:1134-1141.
5. Cassagnol M, Cohen H. Dermatologic toxicities of chemotherapeutic agents. US Pharm. 2008;33(1)(Oncology suppl):10-20.
6. Schulmeister L. Extravasation management. Semin Oncol Nurs. 2007;23:184-190.
7. Viale P. Chemotherapy and cutaneous toxicities: implications for oncology nurses. Semin Oncol Nurs. 2006;22:144-151.
8. Cox K, Stuart-Harris R, Abdini G, et al. The management of cytotoxic-drug extravasation: guide-lines drawn up by a working party for the Clinical Oncological Society of Australia. Med J Aust. 1988;148:185-189.
9. Schrijvers DL. Extravasation: a dreaded complication of chemotherapy. Ann Oncol. 2003;14(suppl 3):iii26-iii30.
10. The National Extravasation Information Service. www.extravasation.org.uk/home.
11. Lemmers N, Gels M, Sleijfer D, et al. Complications of venous access ports in 132 patients with disseminated testicular cancer treated with polychemotherapy. J Clin Oncol. 1996;14:2916-2922.
12. Shetty PC, Mody MK, Kastan DJ. Outcome of 350 implanted chest ports placed by interventional radiologists. J Vasc Interv Radiol. 1997;8:991-995.
13. Dorr RT. Antidotes to vesicant chemotherapy extravasations. Blood Rev. 1990;4:41-60.
14. Langer SW, Sehested M, Jensen PB. Treatment of anthracycline extravasation with dexrazoxane. Clin Cancer Res. 2000;6:3680-3686.
15. Raymond E, Faivre S, Woynarowski JM, Chaney SG. Oxaliplatin: mechanism of action and antineoplastic activity. Semin Oncol. 1998;2(suppl 5):4-12.
16. Rivory LP. New drugs for colorectal cancer—mechanisms of action. Aust Prescr. 2002;25:108-110. www.australianprescriber.com/
17. Skeel RT. Handbook of Cancer Chemotherapy. 5th ed. New York, NY: Lippincott Williams & Wilkins; 1999.
18. Boyle DM, Engelking C. Vesicant extravasation: myths and realities. Oncol Nurs Forum. 1995;22:57-67.
19. Hadaway LC. Preventing and managing peripheral extravasation. Nursing. 2004;34:66-67.
20. Shulmeister L, Camp-Sorrell D. Chemotherapy extravasation from implanted ports. Oncol Nurs Forum. 2000;27:531-538.
21. Debets JM, Wils JA, Schlangen JT. A rare complication of implanted central-venous access devices: catheter fracture and embolization. Support Care Cancer. 1995;3:432-434.
22. Gorski LA. Central venous access device occlusions. Part 2: Nonthrombotic causes and treatment. Home Healthc Nurse. 2003;21:168-171.
23. Extravasation guidelines 2007. European Oncology Nursing Society. www.cancerworld.org/
24. Rimso-50 (dimethyl sulfoxide) package insert. Lake Forest, IL: Bioniche Pharma USA LLC; 2009.
25. Lawrence HJ, Goodnight SH. Dimethyl sulfoxide limiting tissue damage caused by extravasation of doxorubicin agents. Ann Intern Med. 1983;98:1025.
26. Olver IN, Schwarz MA. Use of dimethyl sulfoxide in limiting tissue damage caused by extravasation of doxorubicin [letter]. Cancer Treat Rep. 1983;67:407-408.
27. Berghammer P, Pohnl R, Baur M, Dittrich C. Docetaxel extravasation. Support Care Cancer. 2001;9:131-134.
28. Ludwig CU, Stoll HR, Obrist R, Obrecht JP. Prevention of cytotoxic drug-induced skin ulcers with dimethyl sulfoxide (DMSO) and alpha-tocopherole. Eur J Cancer Clin Oncol. 1987;23:327-329.
29. Olver IN, Aisner J, Hament A, et al. A prospective study of topical dimethyl sulfoxide for treating anthracycline extravasation. J Clin Oncol.1988;6:1732-1735.
30. Bertelli G. Prevention and management of extravasation of cytotoxic drugs. Drug Saf. 1995;12:245-255.
31. Balazs EA, Laurent TC, Jeanloz RW. Nomenclature of hyaluronic acid. Biochem J. 1986;235:903.
32. Girish KS, Kemparaju K. The magic glue hyaluronan and its eraser hyaluronidase. Life Sci. 2007;80:1921-1943.
33. Dorr T. Antidotes to vesicant chemotherapy extravasations. Blood Rev. 1990;4:41-60.
34. Owen OE, Dellatorre DL, Van Scott EJ, Cohen MR. Accidental intramuscular injection of mechlorethamine. Cancer. 1980;45:2225-2226.
35. Totect (dexrazoxane for injection) package insert. Copenhagen, Denmark: TopoTarget A/S; October 2007.
36. Mouridsen HT, Langer SW, Buter J, et al. Treatment of anthracycline extravasation with Savene (dexrazoxane). Results from two prospective clinical multicenter studies. Ann Oncol. 2007;18:546-550.
To comment on this article, contact rdavidson@jobson.com.





