US Pharm. 2022;47(4):HS8-HS12.
ABSTRACT: Patients with heart failure (HF) who contract coronavirus disease 2019 (COVID-19) are at increased risk for morbidity and mortality. Numerous pathophysiologic mechanisms exist by which COVID-19 infection inflicts cardiovascular damage. For HF patients with reduced ejection fraction (HFrEF), guideline-directed medical therapy should be maintained and optimized in the absence of contraindications. Clinicians should assess currently authorized COVID-19 treatment options prior to initiation. Pharmacists play an essential role in managing patients who have HFrEF and recommending preventive therapy, such as lifestyle modifications and vaccinations, with the goal of optimizing health outcomes during the COVID-19 pandemic.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the cause of coronavirus disease 2019 (COVID-19). As of March 4, 2022, the World Health Organization reported more than 442 million confirmed COVID-19 cases globally, with more than 6 million deaths. The United States has had more than 80 million confirmed cases, with nearly 980,000 deaths.1 Although COVID-19 has negatively impacted various patient populations, patients with heart failure (HF) are especially affected. Numerous studies have shown worse clinical outcomes in patients with underlying HF who are hospitalized with COVID-19.2 This article will focus specifically on the management of HF patients with reduced ejection fraction (HFrEF) who have COVID-19.
Pathophysiologic Mechanisms of Cardiovascular Damage
Angiotensin-converting enzyme 2 (ACE2) is a membrane protein that is highly expressed in the heart, lungs, gastrointestinal tract, and kidneys. ACE2 facilitates the passage of SARS-CoV-2 into the host cells, potentially facilitating organ damage. Inside the cell, the virus uses host ribonucleic acid (RNA)-dependent RNA polymerase to replicate its structural proteins; once new virus is assembled, it is released from the cells. This process can result in damage to or destruction of the host cells.3 Patients with underlying cardiovascular (CV) disease have a high level of ACE2 expression, which may explain the increased SARS-CoV-2–associated cardiac damage seen in these patients.4
Indirect pathophysiologic mechanisms include prolonged bed rest and systemic inflammation, which can lead to coagulation disorders. Hypoxemia, a characteristic sign of COVID-19, involves enhanced oxidative stress with production of reactive oxygen species and subsequent intracellular acidosis, mitochondrial damage, and cell death. Fever and sympathetic stimulation can cause tachycardia, which consequently increases myocardial oxygen consumption. COVID-19 also has been known to elicit the abnormal inflammatory response known as cytokine storm. Lymphocytic endotheliitis, a consequence of inflammation induced by COVID-19, can lead to disseminated intravascular coagulation with small-vessel or large-vessel thrombosis and infarction.3
Clinical Manifestations of COVID-19
Patients with COVID-19 may experience fever, cough, muscle aches, new loss of taste or smell, fatigue, and shortness of breath. Complications include acute cardiac injury, acute respiratory distress syndrome, and multiorgan failure. A rare complication of COVID-19 is multisystem inflammatory syndrome (MIS), in which various organs (e.g., heart, lungs, brain) become inflamed. MIS commonly occurs in patients weeks after infection, and its complications can result in ventricular dysfunction and arrhythmias.3
Clinical Outcomes in HF Patients With COVID-19
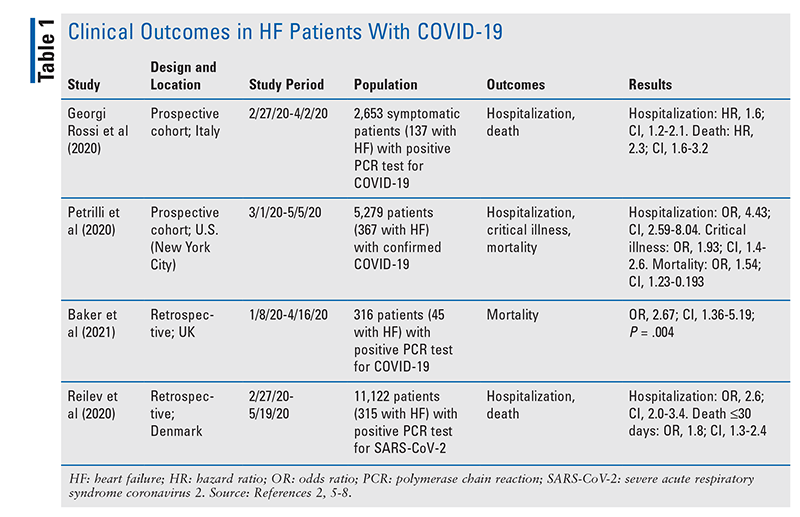
TABLE 1 summarizes several studies from a meta-analysis that showed evidence of worsened clinical outcomes in patients with HF who contracted COVID-19 infection.2,5-8
Pharmacologic Management of HFrEF
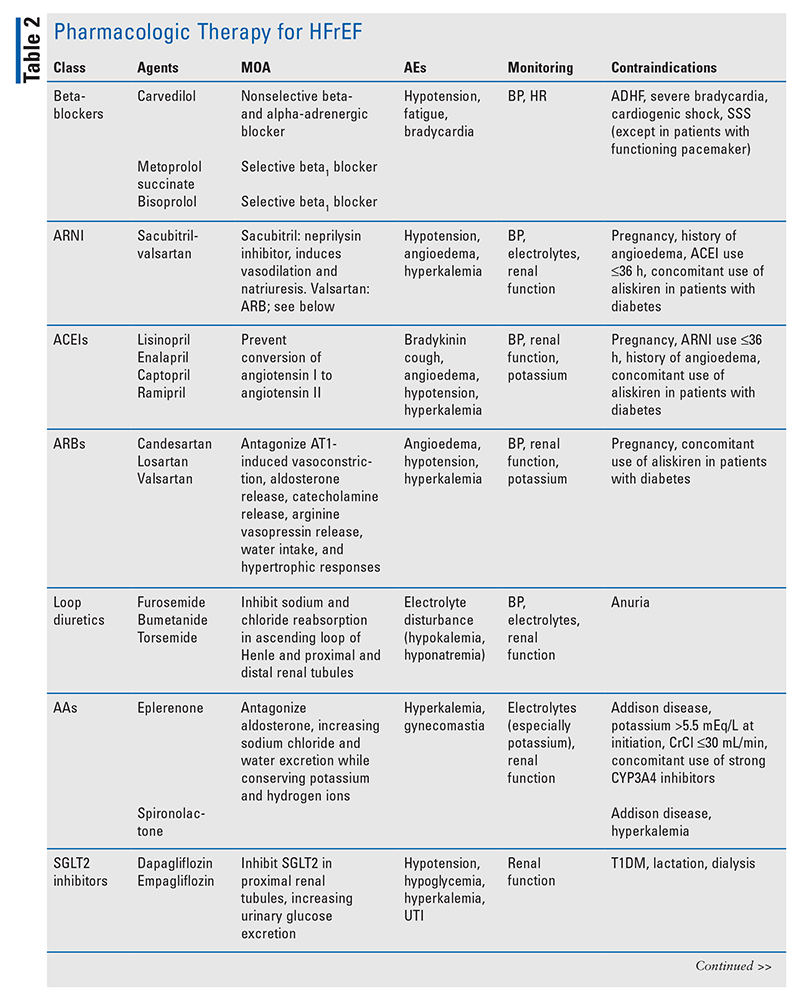
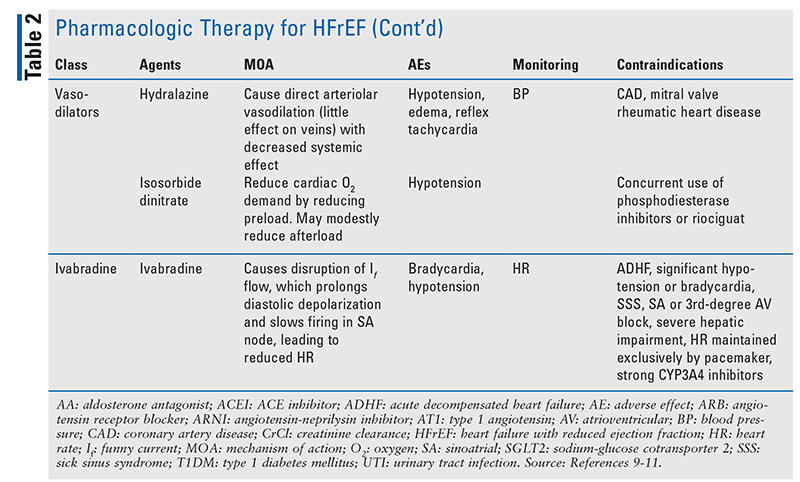
TABLE 2 lists drug classes commonly used to manage HFrEF, as outlined in the American College of Cardiology/American Heart Association/Heart Failure Society of America treatment algorithm.9-11 Guideline-directed medical therapy (GDMT) regimens should be continued in the absence of contraindications.12 Providers should be mindful of possible drug interactions with other medications their patients are taking. Drug interactions and adverse effects (AEs) should be assessed prior to initiation of treatment and throughout the duration of therapy.
Inpatient and Outpatient Management of COVID-19
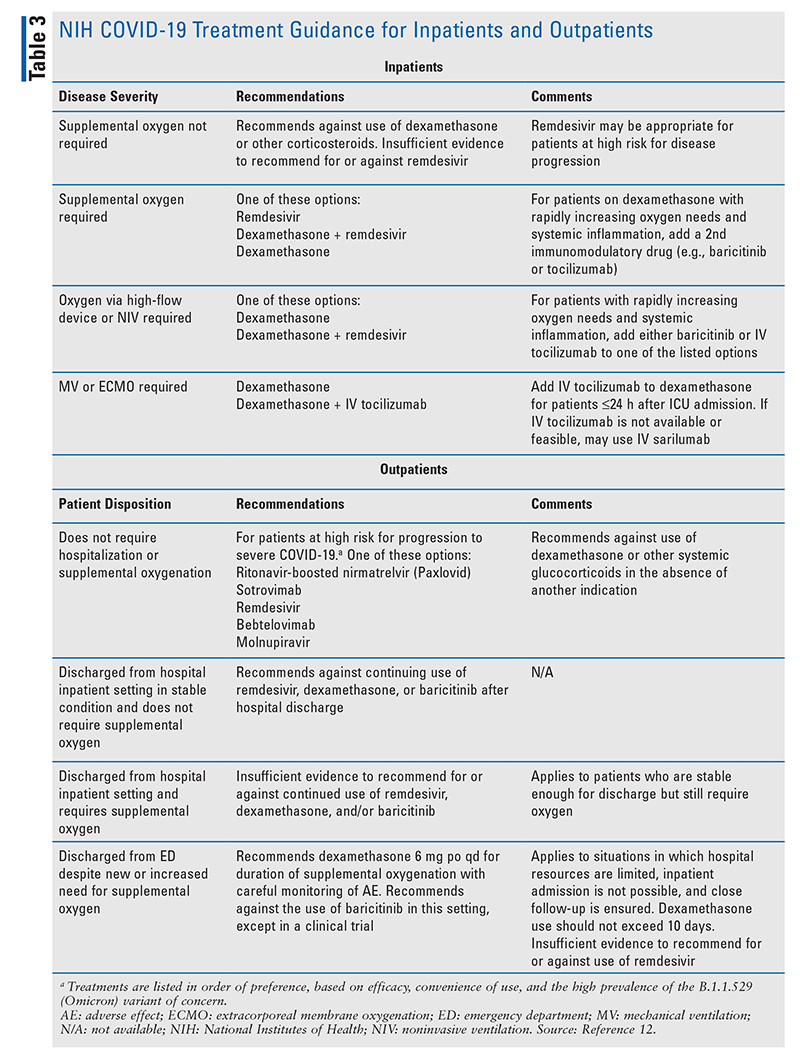
TABLE 3 summarizes the most recent (as of March 4, 2022) COVID-19 treatment guidelines for inpatients and outpatients.12 One update applies to nonhospitalized patients with mild-to-moderate COVID-19 who are at high risk for progressing to severe disease. For these patients, the FDA has granted Emergency Use Authorization (EUA) for the anti–SARS-CoV-2 monoclonal antibody bebtelovimab. The updated guidelines state that either bebtelovimab or molnupiravir may be used as alternative therapy if none of the preferred therapies (nirmatrelvir + ritonavir, sotrovimab, and remdesivir) are available, feasible, or clinically appropriate. It is important to note that clinical data regarding these treatment regimens are limited.12 COVID-19 treatment guidelines are constantly evolving, and clinicians should be cognizant of any updated recommendations before initiating treatment.
Nonpharmacologic Management
Although medications play a crucial role in HFrEF management, it is imperative that patients implement nonpharmacologic preventive measures, such as restricting sodium intake to 2 g to 3 g daily. Daily weight checks are necessary for assessing fluid-overload status. Patients should perform low-to-moderate aerobic exercise (e.g., walking or riding a bike) when possible, with a goal of 30 to 45 minutes of physical exercise daily on most days of the week.13 Patients who smoke should be encouraged to quit, and alcohol intake should be avoided or reduced in order to minimize CV AEs. Pharmacists and other healthcare providers should encourage patients to take advantage of telehealth services when available. In addition, it is crucial to educate patients on the signs and symptoms of worsening HF, such as fatigue, chest pain, weight gain, and orthopnea, and the importance of seeking medical attention when they occur.9,13,14
Certain classes of medications are known to worsen CV disease: nonsteroidal anti-inflammatory drugs (e.g., ibuprofen, naproxen), which cause sodium retention and vasoconstriction; thiazolidinediones (e.g., pioglitazone, rosiglitazone), which cause fluid retention; anthracyclines (e.g., daunorubicin, doxorubicin), which cause myocardial cell injury; and ergotamines (e.g., ergotamine, dihydroergotamine), which cause excessive serotonin activity that can lead to vascular damage.15 These medications should be avoided when possible.15
Vaccine Recommendations
Patients with HF have an increased risk of infection-related morbidity and mortality.3 Vaccines can prevent or reduce illness severity; therefore, clinicians should encourage eligible patients to get vaccinated.16 TABLE 4 lists vaccines specific to COVID-19 as well as some additional vaccines the CDC recommends for patients with chronic heart conditions, including HF.17,18
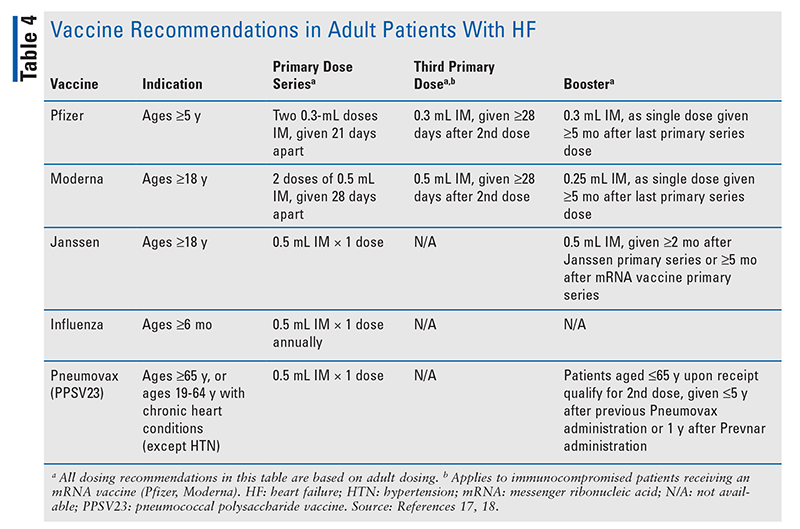
Currently, three COVID-19 vaccines are available.17 Pfizer received FDA approval for use in patients aged 16 years and older and was granted EUA for patients aged 5 years and older. The Moderna and Janssen vaccines have received EUA for patients aged 18 years and older. For the messenger RNA vaccines (Pfizer, Moderna), a third primary dose may be administered to eligible patients who are immunocompromised. Common AEs include local injection-site reactions (arm soreness, redness, swelling), fatigue, headache, and fever. After receiving the vaccine, patients should be monitored for at least 10 minutes for potentially serious AEs (difficulty breathing, urticaria, angioedema).11,16,17
Conclusion
HF patients with COVID-19 are at increased risk for morbidity and mortality. GDMT should be continued and optimized in the absence of contraindications, and lifestyle modifications should be recommended to complement pharmacologic therapy. Numerous COVID-19 treatment options exist, and monoclonal antibodies and antiviral medications have received EUAs. Clinicians should consult updated CDC and National Institutes of Health guidance to determine which patients are appropriate candidates for therapy. Three COVID-19 vaccines with evidence of reducing disease severity are currently available, and patients should also receive routine vaccinations to further protect against preventable diseases. Pharmacists play an essential role in the pharmacotherapeutic management of patients with HFrEF and in recommending preventive therapies (e.g., lifestyle modifications and vaccinations), with the goal of optimizing health outcomes during the COVID-19 pandemic.
REFERENCES
1. Worldometer. COVID live—coronavirus statistics. www.worldometers.info/coronavirus/. Accessed March 4, 2022.
2. Yonas E, Alwi I, Pranata R, et al. Effect of heart failure on the outcome of COVID-19—a meta analysis and systematic review. Am J Emerg Med. 2021;46:204-211.
3. Italia L, Tomasoni D, Bisegna S, et al. COVID-19 and heart failure: from epidemiology during the pandemic to myocardial injury, myocarditis, and heart failure sequelae. Front Cardiovasc Med. 2021;8:713560.
4. Farshidfar F, Koleini N, Ardehali S. Cardiovascular complications of COVID-19. JCI Insight. 2021;6(13:)e148980.
5. Georgi Rossi P, Marino M, Formisano D, et al. Characteristics and outcomes of a cohort of COVID-19 patients in the Province of Reggio Emilia, Italy. PLoS ONE. 2020;15(8):e0238281.
6. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966.
7. Baker KF, Hanrath AT, van der Loeff IS, et al. COVID-19 management in a UK NHS Foundation Trust with a High Consequence Infectious Diseases centre: a retrospective analysis. Med Sci (Basel). 2021;9(1):6.
8. Reilev M, Kristensen KB, Pottegard Å, et al. Characteristics and predictors of hospitalization and death in the first 11 122 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: a nationwide cohort. Int J Epidemiol. 2020;49(5):1468-1481.
9. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599-3726.
10. Hollenberg SM, Warner Stevenson L, Ahmad T, et al. 2019 ACC Expert Consensus Decision Pathway on risk assessment, management, and clinical trajectory of patients hospitalized with heart failure: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2019;74(15):1966-2011.
11. Lexi-Drugs. Hudson, OH: Lexi-Comp, Inc; 2022. https://online.lexi.com. Accessed January 25, 2022.
12. National Institutes of Health COVID-19 Treatment Guidelines. What’s new in the guidelines. www.covid19treatmentguidelines.nih.gov/about-the-guidelines/whats-new/. Accessed March 4, 2022.
13. Mayo Clinic. Heart failure: symptoms & causes. www.mayoclinic.org/diseases-conditions/heart-failure/symptoms-causes/syc-20373142. Accessed November 25, 2021.
14. Cleveland Clinic. Heart failure: exercise and activity. https://my.clevelandclinic.org/departments/heart/patient-education/recovery-care/heart-failure/exercise-activity. Accessed December 25, 2021.
15. Page RL II, O’Bryant CL, Cheng D, et al. Drugs that may cause or exacerbate heart failure. A scientific statement from the American Heart Association. Circulation. 2016;134(6):e32-e69.
16. CDC. Heart disease, stroke, or other cardiovascular disease and adult vaccination. www.cdc.gov/vaccines/adults/rec-vac/health-conditions/heart-disease.html. Accessed January 25, 2022.
17. CDC. Use of COVID-19 vaccines in the United States: interim clinical considerations. www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html. Accessed January 25, 2022.
18. CDC. Adult immunization schedule. www.cdc.gov/vaccines/schedules/hcp/imz/adult.html. Accessed January 23, 2022.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





