US Pharm. 2016;41(4):HS15-HS20.
ABSTRACT: Invasive aspergillosis (IA) is a rare, serious fungal infection commonly affecting immunocompromised patients. The precise incidence of IA has not been documented, and reported incidence rates vary widely. Some typical risk factors for IA include hematopoietic stem-cell transplants, long-term corticosteroid therapy, hematologic malignancies, and HIV. The lungs are the most frequent site of IA; consequently, symptoms and clinical manifestations are typically pulmonary. Because of the high risk of fatality, IA management with the appropriate agents must commence as quickly as possible. Voriconazole is the first-line empirical therapy recommended by Infectious Diseases Society of America guidelines; if there is no clinical response, then salvage therapy with other azoles, echinocandins, or amphotericin B lipid formulations should be administered.
Aspergillosis is a fungal infection caused by Aspergillus, which comprises a large group of ubiquitous mold species (spp) most frequently found in decomposing vegetation.1 Aspergillosis, which is most commonly observed in immunocompromised persons, is a significant cause of morbidity and mortality in this population, with mortality rates as high as 90%.2,3 Several types of aspergillosis infections exist, including allergic bronchopulmonary aspergillosis, allergic Aspergillus sinusitis, aspergilloma, chronic pulmonary aspergillosis, cutaneous aspergillosis, and invasive aspergillosis (IA). Most of these infections are caused by Aspergillus fumigatus, followed by Aspergillus flavus, Aspergillus niger, and Aspergillus terreus.4-6 IA, in particular, is one of the most severe aspergillosis infections, and it has a high mortality rate.5
Etiology and Epidemiology
Common risk factors for IA include hematopoietic stem-cell transplant (HSCT), prolonged and severe neutropenia, hematologic malignancies, long-term corticosteroid therapy, and HIV. However, certain ICU-immunocompetent populations, such as patients with chronic obstructive pulmonary disease, severe liver disease, or critical illnesses that temporarily disrupt the immune system, are also at increased risk for IA.5 The incidence of IA in a typical ICU is reported to be 0.33% to 6.9%, which is comparable to the incidence in solid-organ transplant patients.4,5
Aspergillosis is not a reportable infection in the United States because it is uncommon and not considered a serious threat to public health.6 As a result, the exact incidence of IA has not been documented. Additionally, reported incidence rates can vary substantially, suggesting that geography may play a role.4 Among transplant recipients, the incidence of IA varies, with common underlying conditions including lymphoma and leukemia.7 Among solid-organ transplant recipients, the incidence of IA is higher in lung- and liver-transplant recipients (2.4%-6% and 1%-8%, respectively) than in kidney- and heart-transplant recipients (0.1%-4% and 0.3%-6%, respectively).4 The incidence of IA in HSCT recipients is even higher. Allogeneic HSCT recipients have an IA incidence of 2.3% to 11%, which is more than twice the observed incidence rate in autologous HSCT recipients (0.5%-4%).4 The overall case-fatality rate has been calculated to be 58%.7,8 However, case-fatality rates of IA vary depending on the underlying condition. In a systematic review of 53 studies from 1995 through 1999, the case-fatality rate in IA patients was highest in patients with a history of bone marrow transplants (86.7%) or HIV/AIDS (85.7%) and lowest in patients with leukemia or lymphoma (49.3%).7
Pathogenesis
Following the inhalation of A fumigatus conidia (airborne spores), respiratory epithelial cells and alveolar macrophages trigger innate immune responses against inhaled Aspergillus conidia. These pathogens then recognize receptors on host cells, such as the beta-glucan receptor Dectin-1. The interaction between pathogens and receptors provokes chemokines and cytokines and activates and recruits monocytes and neutrophils. Neutrophils attach to fungal hyphae and degranulate, resulting in fungal killing. This attack is mediated by nicotinamide adenine dinucleotide phosphate oxidase. In the case of neutropenia, neutrophils are not available for hyphal killing and the control of fungal growth, so growing hyphae can then spread throughout the endothelial cell lining.8
Clinical Manifestations
The lungs are the most common site of IA. Consequently, IA symptoms and clinical manifestations typically are pulmonary. In one study, Lee and colleagues observed IA symptoms in 36 patients and found that hemoptysis was the most common clinical manifestation (72%), followed by cough, shortness of breath, and fatigue.9 The investigators noted that IA symptoms ranged from asymptomatic to massive hemoptysis (sometimes fatal), and the rate of symptom onset varied based on the patient’s immune system. Patients with some degree of immunosuppression (e.g., AIDS, corticosteroid use, diabetes mellitus, alcoholism) may present with more rapid development of symptoms (i.e., weeks rather than months).10 In a 6-year survey conducted by Cornillet and colleagues, fever was the most common clinical sign in IA patients, presenting in 85% of patients (n = 88); chest pain was common as well.11 Additional symptoms include weight loss, sweats, and anorexia.10
Diagnosis
Culturing of tissue is currently the gold standard for the diagnosis of IA.11 This method not only yields Aspergillus spp, but further defines therapeutic options via susceptibility testing. However, the invasiveness of obtaining tissue makes culturing less desirable. In the absence of tissue specimens, bronchoalveolar lavage (BAL) fluid obtained from the upper and lower respiratory tracts can serve to establish the diagnosis of IA.12 Although BAL is a safe procedure, the overall sensitivity of culture using BAL specimens is relatively low (estimated at 50%).13 Blood sampling is the optimal noninvasive diagnostic approach for IA.12 Despite this noninvasiveness, however, Aspergillus spp are rarely isolated from blood through conventional culture techniques—hence the reliance on tissue specimens to secure a definitive diagnosis of IA.12 Since results from cultures are relatively slow to obtain overall, IA is well established by the time the culture is positive. These challenges have resulted in the development of diagnostic methods that are less invasive and more rapid.
The serum galactomannan assay is a rapid test that is approved to diagnose suspected cases of IA in patients with hematologic malignancies or HSCTs.13 Galactomannan, a polysaccharide released in bodily fluids during Aspergillus growth, can be detected in serum and BAL specimens several days before clinical signs and symptoms appear.13 Serum detection may facilitate the establishment of a specific diagnosis, whereas BAL fluid appears to have a higher sensitivity but is often harder to obtain.12,13 Meersseman and colleagues reported the sensitivity of galactomannan detection to be 88% for BAL versus 42% for serum, and the specificity of galactomannan for both BAL and serum was 86%.14 A positive serum result can enable the initiation of antifungal agents, whereas a negative result can potentially prevent the use of expensive and toxic antifungal drugs.12 Measurement of (1,3)-beta-D-glucan in the blood may also be useful as a screening tool for IA. Although the galactomannan assay is specific for IA, the (1,3)-beta-D-glucan assay also can detect other invasive fungal diseases, including candidiasis, other mold pathogens, and Pneumocystis jiroveci.15
Management
Antifungal treatment remains the mainstay of treatment of IA.2 In order to prevent complications and poor outcomes, antifungal treatment should be initiated immediately in patients at high risk for IA.2 The Infectious Diseases Society of America (IDSA) recommends the use of voriconazole for the primary treatment of invasive pulmonary aspergillosis, as well as for other manifestations, such as aspergillosis infections of the sinuses, central nervous system aspergillosis, and chronic necrotizing pulmonary aspergillosis.2 If primary treatment fails, lipid formulations of amphotericin B (AMB), caspofungin, and itraconazole may be used as salvage therapy.2 The IDSA guidelines also recommend the use of posaconazole for prophylaxis in neutropenic patients with myelodysplasia and leukemia, as well as in allogeneic HSCT recipients with graft-versus-host disease (GvHD).2
Empirical Treatment
Voriconazole: A randomized, nonblinded trial comparing voriconazole with AMB found that 52.8% of voriconazole patients experienced successful outcomes (complete or partial resolution of lesions on radiology).16 The voriconazole group also had a higher survival rate than AMB patients (70.8% vs. 57.9%) and fewer adverse events (343 events vs. 421 events; P = .02), leading investigators to conclude that voriconazole is superior to AMB.16 The voriconazole dosage recommended by the IDSA guidelines (TABLE 1) was supported in this trial.2 Voriconazole has an adverse-effect profile that includes skin sensitivity with exposure to direct sunlight, visual disturbances, and hepatotoxicity characterized by an increase in serum bilirubin and liver enzymes.2
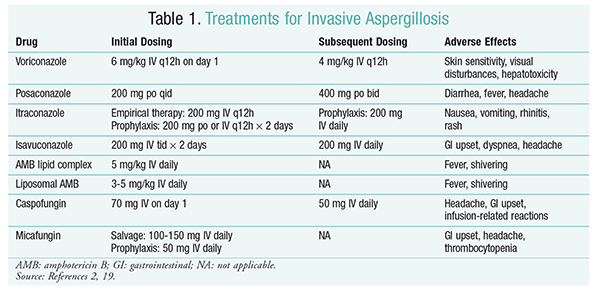
AMB: Previously, AMB deoxycholate was the standard of care for aspergillosis. However, infusion-related reactions (including fever, rigors, arthralgias, vomiting, and bronchospasms) and nephrotoxicity resulting in potential renal failure have caused AMB to fall out of favor.2 Additionally, azotemia may occur when AMB deoxycholate is coadministered with nephrotoxic agents (e.g., cyclosporine or tacrolimus).2 As a result, the IDSA recommends lipid complex formulations as an alternative, as the incidence of adverse effects is lower than that for the deoxycholate formulation. Moreover, in a trial by Walsh and colleagues that compared AMB lipid complex with caspofungin, success rates were similar (33.7% for AMB, 33.9% for caspofungin); therefore, the treatment guidelines list both AMB lipid complex and caspofungin as alternatives to voriconazole.17
Echinocandins (Caspofungin, Micafungin, Anidulafungin): Although caspofungin has been approved for IA therapy, micafungin and anidulafungin have not proven efficacious in the primary treatment of IA and therefore are not recommended by the IDSA.18 Caspofungin may cause headache, gastrointestinal upset, and possible infusion-related reactions owing to histamine release.2
Isavuconazole: In March 2015, the FDA approved a novel second-generation triazole for IA. Marketed under the trade name Cresemba, isavuconazole is available in both oral and IV solutions and is indicated for the treatment of IA and mucormycosis.19 In a comparison of isavuconazole and voriconazole, the SECURE study determined that the novel agent performed similarly with regard to all-cause mortality and overall response at the end of therapy. The primary endpoint of all-cause mortality by day 42 was 19% for isavuconazole and 20% for voriconazole.20 However, isavuconazole was deemed noninferior since the investigators used a 10% noninferiority margin (CI –7.8 to 5.7). Isavuconazole had fewer adverse events than voriconazole, particularly events involving the eyes, skin, and hepatobiliary system.19 Finally, isavuconazole has high oral bioavailability, yielding a 1:1 ratio in a conversion between IV and oral formulations.
Salvage Therapy
The IDSA guidelines do not recommend combination therapy for the initial treatment of IA. Instead, salvage therapy is recommended only if there is no clinical response to initial therapy. Consequently, this may involve adding a second agent or switching to another medication. Posaconazole, itraconazole, AMB, and caspofungin have been studied for use as salvage therapy and are recommended by IDSA guidelines. Walsh and colleagues investigated the efficacy and safety of posaconazole 800 mg suspension daily in patients who were intolerant or nonresponsive to the initial antifungal agent.21 The overall success rate was 42% for the posaconazole group and 26% for the control group (P = .006).21 A study by Ng and Denning demonstrated a 40% response rate when a lipid-based formulation of AMB was used as salvage therapy in IA patients who were refractory or intolerant to existing therapy.22 Caspofungin was also examined as salvage therapy in a noncomparative multicenter study of patients who were refractory to conventional therapy; a favorable response occurred in 44.6 % of patients.23
Prophylaxis
Posaconazole: The IDSA guidelines recommend the use of posaconazole for prophylaxis of IA. This recommendation is based on two randomized, controlled, multicenter trials that evaluated the prophylactic use of posaconazole in patients with neutropenia and GvHD.24,25 The first trial compared posaconazole (3 × 200 mg/day) with fluconazole (1 × 400 mg) or itraconazole (2 × 200 mg). IA was reported in 1% of patients in the posaconazole group versus 7% of patients in the fluconazole and itraconazole groups (P <.001).26 The second study compared posaconazole with fluconazole for prophylaxis in patients with GvHD. IA was less frequent in the posaconazole group than in the fluconazole group (1.0% vs. 5.9%; P = .001).25
Micafungin: Although not approved for first-line prophylaxis of IA, the IDSA guidelines recommend micafungin as an alternative to posaconazole. This recommendation is based on a randomized, double-blind, phase III trial of 882 patients who received micafungin 50 mg IV (1 mg/kg in patients <50 kg) versus fluconazole 400 mg (8 mg/kg in patients <50 kg). Efficacy was defined as an absence of proven, probable, or suspected systemic fungal infection. During the neutropenic phase, the overall efficacy of micafungin was superior to that of fluconazole (80% for the micafungin group vs. 73.5% for the fluconazole group; 95% CI, 0.9%-12%; P = .03).27
Conclusion
IA is a severe fungal infection that occurs most commonly in immunocompromised patients. Several questions need to be addressed in the diagnosis, treatment, and prevention of aspergillosis. Diagnostic criteria are poorly defined, rendering the diagnosis of IA challenging. Owing to the high risk of fatality, antifungal therapy must be initiated swiftly and correctly in order to be effective. Critical gaps exist regarding the use of combination therapy, tools for early detection, and the patient populations in whom prophylaxis would be most beneficial.2 Therefore, it is important for pharmacists to be knowledgeable about each antifungal agent’s characteristics in order to be best equipped to make sound therapeutic decisions in the treatment of IA. Indeed, given the increased risk of antifungal resistance, pharmacists can play an important role in the management of IA by assisting in the minimization and management of adverse effects, making appropriate dosing recommendations, and monitoring the selection of suitable antifungal agents—all of which are critical to optimizing therapy for IA.
REFERENCES
1. Denning DW. Invasive aspergillosis. Clin Infect Dis. 1998;26:781-803.
2. Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:327-360.
3. Kousha M, Tadi R, Soubani AO. Pulmonary aspergillosis: a clinical review. Eur Respir Rev. 2011;20:156-174.
4. Barnes PD, Marr KA. Aspergillosis: spectrum of disease, diagnosis, and treatment. Infect Dis Clin North Am. 2006;20:545-561,vi.
5. Dimopoulos G, Frantzeskaki F, Poulakou G, Armaganidis A. Invasive aspergillosis in the intensive care unit. Ann N Y Acad Sci. 2012;1272:31-39.
6. Aarabi MH, Kamalian A, Mohajer B, et al. A statistical approach in human brain connectome of Parkinson disease in elderly people using Network Based Statistics. Conf Proc IEEE Eng Med Biol Soc. 2015;2015:4310-4313.
7. Lin SJ, Schranz J, Teutsch SM. Aspergillosis case-fatality rate: systematic review of the literature. Clin Infect Dis. 2001;32:358-366.
8. Dagenais TR, Keller NP. Pathogenesis of Aspergillus fumigatus in invasive aspergillosis. Clin Microbiol Rev. 2009;22:447-465.
9. Lee SH, Lee BJ, Jung DY, et al. Clinical manifestations and treatment outcomes of pulmonary aspergilloma. Korean J Intern Med. 2004;19:38-42.
10. Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax. 2015;70:270-277.
11. Cornillet A, Camus C, Nimubona S, et al. Comparison of epidemiological, clinical, and biological features of invasive aspergillosis in neutropenic and nonneutropenic patients: a 6-year survey. Clin Infect Dis. 2006;43:577-584.
12. Musher B, Fredricks D, Leisenring W, et al. Aspergillus galactomannan enzyme immunoassay and quantitative PCR for diagnosis of invasive aspergillosis with bronchoalveolar lavage fluid. J Clin Microbiol. 2004;42:5517-5522.
13. Wheat LJ, Walsh TJ. Diagnosis of invasive aspergillosis by galactomannan antigenemia detection using an enzyme immunoassay. Eur J Clin Microbiol Infect Dis. 2008;27:245-251.
14. Meersseman W, Vandecasteele SJ, Wilmer A, et al. Invasive aspergillosis in critically ill patients without malignancy. Am J Respir Crit Care Med. 2004;170:621-625.
15. Sherif R, Segal BH. Pulmonary aspergillosis: clinical presentation, diagnostic tests, management and complications. Curr Opin Pulm Med. 2010:16:242-250.
16. Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408-415.
17. Walsh TJ, Teppler H, Donowitz GR, et al. Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. N Engl J Med. 2004;351:1391-1402.
18. Denning DW, Marr KA, Lau WM, et al. Micafungin (FK463), alone or in combination with other systemic antifungal agents, for the treatment of acute invasive aspergillosis. J Infect. 2006;53:337-349.
19. Pettit NN, Carver PL. Isavuconazole: a new option for the management of invasive fungal infections. Ann Pharmacother. 2015;49:825-842.
20. Maertens JA, Raad II, Marr KA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet. 2016;387:760-769.
21. Walsh TJ, Raad I, Patterson TF, et al. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin Infect Dis. 2007;44:2-12.
22. Ng TT, Denning DW. Liposomal amphotericin B (AmBisome) therapy in invasive fungal infections. Evaluation of United Kingdom compassionate use data. Arch Intern Med. 1995;155:1093-1098.
23. Maertens J, Raad I, Petrikkos G, et al. Efficacy and safety of caspofungin for treatment of invasive aspergillosis in patients refractory to or intolerant of conventional antifungal therapy. Clin Infect Dis. 2004;39:1563-1571.
24. Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356:348-359.
25. Ullmann AJ, Lipton JH, Vesole DH, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007;356:335-347.
26. Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356:348-359.
27. van Burik JA, Ratanatharathorn V, Stepan DE, et al. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis. 2004;39:1407-1416.
To comment on this article, contact rdavidson@uspharmacist.com.






