US Pharm. 2022;47(12):HS-1-HS-6.
ABSTRACT: Acute gastrointestinal (GI) bleeds account for numerous patient visits to the emergency department and subsequent hospitalization. Depending on anatomical location, initial management is largely the same. Once the type and source of the GI bleed is identified, targeted therapy may be initiated. Pharmacists are posed to ensure that proper targeted medical treatment is initiated, assist with de-escalation of therapies when appropriate, and advise on reversal and reinitiation of antithrombotic agents.
Gastrointestinal (GI) hemorrhage is the most common GI diagnosis and accounts for more than 500,000 hospitalizations in the United States.1 Acute GI bleeds are split into two categories depending on the origin: upper GI bleeds (UGIBs) and lower GI bleeds (LGIBs). Risk factors for developing these potentially fatal bleeds include alcoholism, chronic nonsteroidal anti-inflammatory drug (NSAID) use, and chronic or forceful emesis. UGIBs originate from the esophagus, stomach, or duodenum, and LGIBs encompass the duodenojejunal flexure to the rectum.2 Patient presentation differs with respect to the type of bleed and will guide initial management. Initial management is similar for upper and lower GI bleeds; therefore, further evaluation to find the source of the bleed is necessary to initiate targeted therapy based on the etiology.2,3
Acute UGIB
Patients with UGIBs often present with signs of hematemesis and melena.4 Hematemesis is defined as presence of blood in vomit and can be described as either bright red blood or coffee ground in appearance. Melena is the presence of digested blood in the stool, which is described as black and tarry in appearance. In severe cases, it is possible for patients to present with hematochezia (bright red blood in stool) similar to that found in acute LGIBs. Common causes of UGIBs include ulcers, esophageal varices, and Mallory-Weiss tears.5 It is beneficial to inquire about the patient’s history of GI bleeds to evaluate for the possibility of recurrence.
Ulcers
Peptic (esophageal, gastric, and duodenal) ulcers comprise approximately 65% of UGIBs.5 Esophageal ulcer formation largely results from esophagitis due to repeated exposure to gastric acid, often due to gastroesophageal reflex disease (GERD).6 The two primary causes of gastric and duodenal ulcers are Helicobacter pylori infection and chronic NSAID use.7 Both of these etiologies damage the protective mucosal epithelium of the stomach and duodenum, allowing acidic parietal cell secretions to erode subsequent layers of gastric tissue.5 Symptoms include nausea, heartburn, bloating, and burning stomach pain, and if left untreated, the ulcers can develop into UGIBs.8
Mallory-Weiss Tear
Mallory-Weiss tears (MWTs) comprise approximately 7% of all UGIBs.5 An MWT is depicted as a laceration that extends longitudinally along the mucosa of the gastroesophageal junction.9 Risk factors for developing an MWT are increasing age, history of alcoholism, forceful vomiting, and other activities that may cause strain and increased abdominal pressure (e.g., lifting, blunt trauma).10
Esophageal Varices
Esophageal varices are the final etiology of UGIBs that will be highlighted in this review. When the veins that line the esophagus become engorged, they are termed esophageal varices.11 This condition is primarily associated with chronic liver failure and results from reduced blood flow through the liver that causes increased pressure in the portal vein. Portal vein hypertension causes an accumulation of blood in the veins of the esophagus, leading to venous engorgement. Esophageal varices may not result in symptoms until these veins rupture, resulting in an UGIB.
Management of UGIB
FIGURE 1 describes the management of UGBIs.
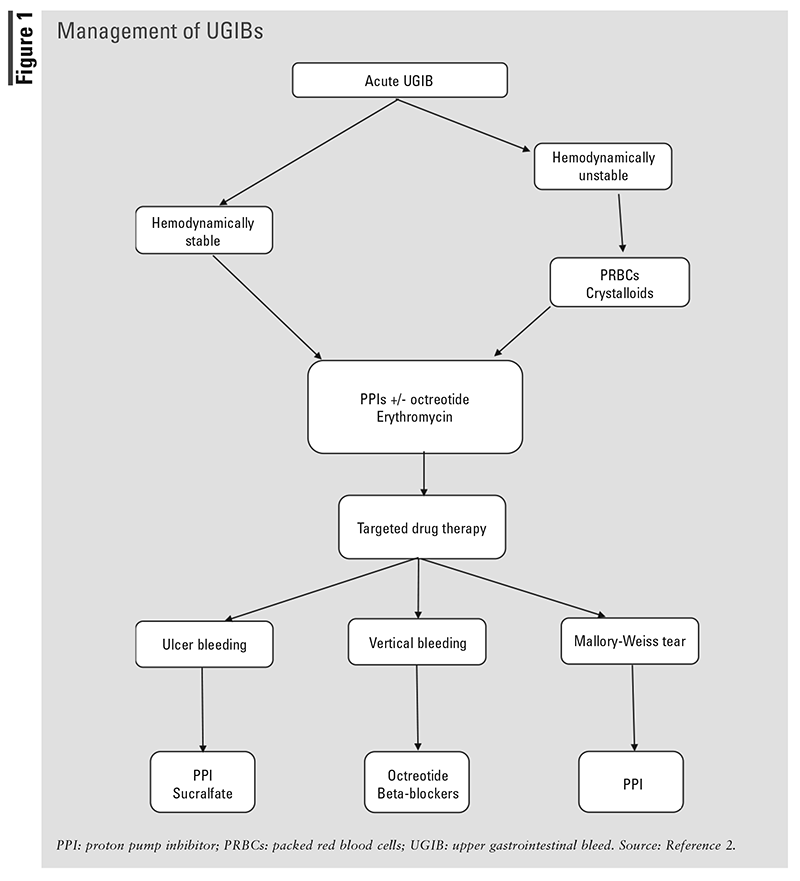
Initial Management
Upon initial suspicion of a UGIB, hemodynamically unstable patients should be evaluated for administration of crystalloid fluids and/or packed red blood cells (PRBCs).2 Acid-suppressive agents are initiated, along with vasoactive medications in cases of suspected variceal bleeding. An upper endoscopy is the cornerstone for confirming a UGIB and determining its location. Guidelines suggest performing endoscopy within 24 hours of presentation.
PRBC
Guidelines published in 2021 by the American College of Gastroenterology (ACG) set restrictive standards for PRBC transfusions, defined as serum hemoglobin (Hgb) of £7 g/dL or Hgb £8 g/dL in the presence of hypotension.2 Transfusion of 1 unit is recommended for most patients; however, the need for more than 1 unit is dependent upon the degree of anemia and severity of the bleed.12
GI Motility Agents
In preparation for endoscopy, GI motility agents such as erythromycin and metoclopramide may be administered 20 to 90 minutes prior to allow clear visualization of the lesion.2,4 Erythromycin’s prokinetic properties can be attributed to its motilin agonism promoting GI peristalsis, while metoclopramide increases sensitivity to acetylcholine in the GI tract.13,14 Guidelines from ACG recommend an IV erythromycin infusion of 250 mg given over 20 to 30 minutes.2,4 While metoclopramide may be substituted in practice, guidelines do not recommend its use due to limited evidence of efficacy versus erythromycin’s proven effectiveness.2,14,15 When utilized, the dose of metoclopramide should be 10 mg IV administered 30 to 60 minutes prior to endoscopy.
Proton Pump Inhibitors
Proton pump inhibitors (PPIs), such as pantoprazole and esomeprazole, suppress acid secretion by irreversibly binding the H+/K+ ATPase in the parietal cells of the gastric epithelium.16 The reduction of gastric acid is hypothesized to induce clotting. While PPIs are a mainstay of postendoscopic treatment, a recommendation for their use pre-endoscopy has not been concluded due to conflicting evidence.2 Although pre-endoscopic PPIs are a topic of debate, ACG guidelines recommend the use of high-dose PPIs for 3 days after a bleeding ulcer has been successfully treated with endoscopic hemostasis. The high-dose regimen includes an 80-mg IV bolus followed by an 8-mg/hour continuous infusion. Alternatively, the 80-mg bolus may be followed by intermittent dosing of 40 mg IV two to four times daily for 3 days.
Octreotide
Octreotide, an analogue of somatostatin, is a natural hormone that regulates the release of serotonin (5-HT) and other physiologic chemicals like gastrin, glucagon, insulin, and growth hormone.17 Commonly used as targeted therapy for variceal bleeds, octreotide can be used in the initial management of other UGIB sources if endoscopy is not feasible.8 Additionally, octreotide may help with a GI bleed caused by an ulcer through a reduction of splanchnic blood flow and gastric acid secretion, as well as providing gastric cytoprotection. Octreotide is initiated as a 50-mcg IV bolus, followed by a 50-mcg/hour continuous infusion. The 50-mcg IV bolus may be repeated in the first hour for uncontrolled hemorrhage.18
Targeted Drug Therapy
Peptic Ulcers
PPIs: After the initial 3-day postendoscopic regimen is completed, a twice-daily maintenance dose of IV or PO PPI should be continued from Days 4 to 14 of treatment.2 After the initial 14-day treatment period, patients may be switched to once-daily dosing for an additional 2 to 6 weeks depending on factors such as ulcer size, location, and complexity.19,20 It should be noted that patients with recurrent or refractory ulcer bleeding may be continued on twice-daily dosing indefinitely.6
Sucralfate: Sucralfate promotes healing of duodenal ulcers by binding to the ulcer and creating a protective barrier.21 The protective layer of sucralfate thwarts potential damage from gastric secretion as well as ingested irritants (e.g., alcohol, NSAIDs). The initial dosing regimen for the treatment of duodenal ulcers includes 1 g of oral sucralfate suspension four times a day for up to 8 weeks followed by a maintenance dose of 1 g twice daily. Gastric ulcers may also be treated with sucralfate even though efficacy for this indication has yet to be validated. It is important to recognize that sucralfate contains aluminum; therefore, patient counseling is pertinent, especially for those with renal impairment or receiving dialysis. Sucralfate has the potential to adsorb other medications, leading to a reduction in bioavailability. Some examples of these medications are ciprofloxacin, tetracycline, phenytoin, ketoconazole, and digoxin. Sucralfate doses should be separated from these other medications by at least 2 hours.
MWTs
The management of MWTs involves either endoscopic treatment or antisecretory therapy with PPIs, depending on the severity of the lesion.2 Several endoscopic hemostatic treatments are available to treat actively bleeding MWTs. Injection with epinephrine, diluted with saline (1:10,000-1:20,000) is an example of one pharmacologic modality used during endoscopy. This treatment is not used as monotherapy but has proven effective to temporarily slow or pause bleeding due to potent alpha-1 vasoconstriction of the smooth muscle. All patients with MWTs should receive PPI therapy, beginning with a twice-daily regimen prior to endoscopy and de-escalating to a once-daily regimen post endoscopy.10 The duration of PPI therapy is generally limited to 2 weeks.
Esophageal Varices
Octreotide: Octreotide counteracts the effects of variceal hemorrhage by blocking vasodilatory chemical messengers (i.e., 5-HT, glucagon) and decreasing blood flow via splanchnic vasoconstriction.22 It is one of the most utilized drugs worldwide for variceal hemorrhage and should immediately be administered to patients presenting with varices or those with a peptic ulcer bleed at risk of developing varices.22,23 The 2016 guidelines from the American Association for the Study of Liver Diseases (AASLD) recommend continuing the 50-mcg/hour continuous infusion for 2 to 5 days until bleeding is controlled.22 A second bolus may be administered within the first hour if hemostasis is not achieved.
Beta-Blockers: Nonselective beta-blockers are used as primary and secondary esophageal variceal hemorrhage prophylaxis.22 They exert activity by blocking beta-1 adrenergic receptors in the heart and beta-2 receptors in the splanchnic venous system. Beta-1 blockade decreases splanchnic blood flow by decreasing cardiac output, while beta-2 blockade directly promotes splanchnic vasoconstriction. Nadolol, propranolol, and carvedilol are specifically recommended in the AASLD guidelines. The target heart rate is 55 to 60 bpm, and blood pressure monitoring is required with a systolic blood pressure goal of no less than 90 mmHg. Blood pressure monitoring is especially pertinent with carvedilol due to its additional effects on the alpha-1 receptor. Patients should continue beta-blocker therapy indefinitely.
Acute LGIB
Lower gastrointestinal bleeds (LGIBs) occur between the duodenojejunal flexure, an area of the colon affixed to the ligament of Treitz, and the rectum.3 Severe acute LGIBs are mostly caused by diverticulosis, angioectasia, post polypectomy bleeding, and ischemic colitis. As previously mentioned, hematochezia and melena are signs of both UGIBs and LGIBs; therefore, source identification is pertinent.
Diverticulosis
Diverticular bleeding typically presents as painless hematochezia.3 It is caused by increased luminal pressure and weakened colonic wall resistance, which results in saclike protrusions that press against weak areas of the colonic wall.24 Along the mucosal layer of the colon, the vasa recta (artery terminal branches) become exposed and subject to injury as diverticular herniation continues. Injury to the vasa recta induces eccentric intimal-media thickening and thinning of the vessels, ultimately causing segmental arterial weakness and rupture.
Angiodysplasia
Angiodysplasia is described as dilated, thin-walled, tortuous blood vessels that lack smooth muscle.25 The mechanism of angiodysplasia is unclear but is thought to be the result of muscular vein obstruction. It can affect the upper and lower GI tract. In the lower GI tract, angiodysplasia is most frequent in the mucosa and submucosa of the right-sided colon and cecum. Bleeding may be occult or overt, especially in patients who are taking anticoagulants or antiplatelets.3
Ischemic Colitis
Ischemic colitis is the most common intestinal form of ischemia.26 It is caused by a sudden reduction in blood flow, usually in areas with limited collateral blood flow, compromising the nutrient and oxygen delivery needed for cell survival. The origin of hypoperfusion associated with colonic ischemia could be nonocclusive, embolic, or thrombotic in nature, suggesting multifactorial pathogenicity and no specific risk factors. Symptoms that aid in the diagnosis of ischemic colitis include cramping, abdominal pain, urge to defecate, and hematochezia.
Management of LGIB
Initial Management
Hemodynamically unstable patients should be managed similarly to those with UGIBs, utilizing PRBCs and/or crystalloid fluids.3 Colonoscopy should be performed to confirm the presence of an LGIB, with adequate bowel preparation using 4 to 6 L of a polyethylene glycol solution prior to the procedure. Colonoscopy should ideally be performed within 24 hours if a patient presents with high-risk clinical features and an active bleed. Targeted treatments depend on the source of the bleeding and, in many cases, can be administered during the colonoscopy.
Targeted Drug Therapy
Colonic Diverticular Bleeding and Angiodysplasia Rupture: The ACG guidelines recommend endoscopic hemostatic methods for treating colonic diverticular bleeding and angiodysplasia rupture.3 During the management of active bleeds, 0.5-mL to 2-mL injections of epinephrine + saline admixtures (1:10,000 or 1:20,000) can aid in hemostasis and prompt visualization for clip placement. To prevent recurrent bleeds, ACG recommends avoiding nonaspirin NSAIDs.
Ischemic Colitis: Most cases of ischemic colitis resolve spontaneously and do not require targeted intervention.26 The ACG guidelines suggest surgical consultation for patients presenting with hypotension, tachycardia, or abdominal pain. Broad-spectrum antibiotics may be considered in cases of moderate-to-severe colitis. The ACG guideline does not designate a particular antibiotic as most efficacious or how long it should be continued; however, a 72-hour course of anaerobic coverage plus a fluoroquinolone, aminoglycoside, or third-generation cephalosporin is recommended. If the patient shows improvement within 72 hours, a 7-day antimicrobial regimen may be considered.
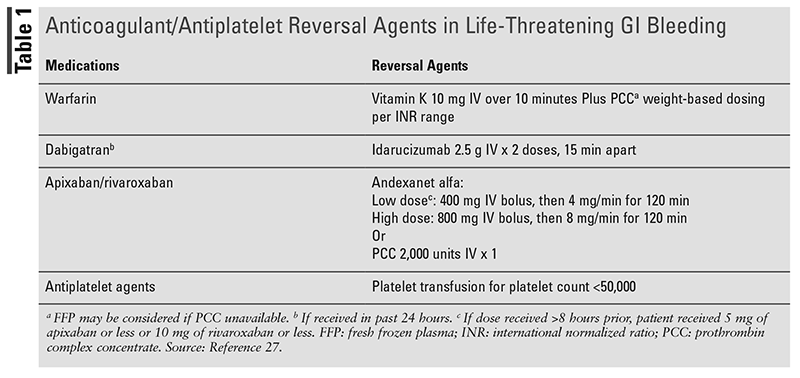
Managing Patients on Anticoagulants and Antiplatelets: In 2022, a guideline-directed dissemination tool was created in collaboration with ACG and the Canadian Association of Gastroenterology to deliver guidance on the management of anticoagulant and antiplatelet medications in the setting of acute GI bleeds.27 These recommendations guide the decision process of selecting a reversal agent and when to resume anticoagulants and antiplatelets post endoscopy. For patients on warfarin or direct-acting anticoagulants with life-threatening bleeds or significantly supratherapeutic international normalized rate, reversal agents should be administered (TABLE 1). In the absence of these factors, recommendations for reversal agents could not be made due to the availability of low levels of evidence. Anticoagulation should be held temporarily with evaluation for appropriateness of resuming post GIB. The guidelines have a conditional recommendation for continuing aspirin when used for secondary prevention. In patients receiving dual-antiplatelet therapy, the P2Y12 inhibitor should be temporarily interrupted with consideration for continuing aspirin therapy.
Pharmacist’s Role
Pharmacists are poised to provide medication stewardship in the management of acute GIBs. While empiric management of acute GIBs is similar regardless of the source, pharmacists can ensure that appropriate targeted therapy occurs once bleeding sources are confirmed. In the setting of UGIBs, pharmacists can assist with de-escalation of octreotide infusions in patients with confirmed nonvariceal bleeding, earlier conversion to oral PPI therapy, and appropriate step-down of twice-daily to once-daily PPI dosing. The lack of recommendations for PPI therapy in LGIBs also serves as an opportunity for PPI de-escalation in this patient population. Once an UGIB has been adequately ruled out, pharmacists may promote discontinuation of unnecessary PPI use. For patients presenting with a GI bleed on an antithrombotic, pharmacists can assist in recommending appropriate reversal agents along with facilitating their dosing, admixture, and appropriate administration. Given their expertise on appropriate medication use, pharmacists are equipped to properly evaluate indications and suitable durations for anticoagulant and antiplatelet therapies to facilitate their timely resumption when necessary.
REFERENCES
1. Peery AF, Crockett SD, Murphy CC, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2018. Gastroenterology. 2018;156(1):254-272.e11.
2. Laine L, Barkun AN, Saltzman JR, et al. ACG clinical guideline: upper gastrointestinal and ulcer bleeding. Am J Gastroenterol. 2021;116(5):899-917.
3. Strate LL, Gralnek IM. ACG clinical guideline: management of patients with acute lower gastrointestinal bleeding. Am J Gastroenterol. 2016;111(4):459-474.
4. Rockey DC. Causes of upper gastrointestinal bleeding in adults. UptoDate. 2022. www.uptodate.com. Accessed September 29, 2022.
5. Wuerth BA, Rockey DC. Changing epidemiology of upper gastrointestinal hemorrhage in the last decade: a nationwide analysis. Dig Dis Sci. 2018;63(5):1286-1293.
6. Bardhan KD. Is there any acid peptic disease that is refractory to proton pump inhibitors? Aliment Pharmacol Ther. 1993;7(s1):13-24.
7. Huang JQ, Sridhar S, Hunt RH. Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic-ulcer disease: a meta-analysis. Lancet. 2002;359(9300):14-22.
8. Saltzman JR. Overview of the treatment of bleeding peptic ulcers. UptoDate. 2022. www.uptodate.com. Accessed September 29, 2022.
9. Michel L, Serrano A, Malt RA. Mallory-Weiss syndrome. Evolution of diagnostic and therapeutic patterns over two decades. Ann Surg. 1980;192(6):716-721.
10. Guelrud M. Mallory-Weiss syndrome. UptoDate. 2022. www.uptodate.com. Accessed September 29, 2022.?
11. Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. 2010;362:823-832.
12. Carson JL. Indications and hemoglobin thresholds for red blood cell transfusion in the adult. UptoDate. 2022. www.uptodate.com. Accessed September 29, 2022.
13. Erythromycin. Lexi-Drugs. Hudson, OH: Lexicomp; 2022. online.lexi.com. Updated September 19, 2022. Accessed September 29, 2022.
14. Metoclopramide. Lexi-Drugs. Hudson, OH: Lexicomp; 2022. online.lexi.com. Updated September 19, 2022. Accessed September 29, 2022.
15. Daram SR, Garretson R. Erythromycin is preferable to metoclopramide as a prokinetic in acute upper GI bleeding. Gastrointest Endosc. 2011;74(1):234.
16. Sreedharan A, Martin J, Leontiadis GI, et al. Proton pump inhibitor treatment initiated prior to endoscopic diagnosis in upper gastrointestinal bleeding. Cochran Database Syst Rev. 2010(7):
CD005415.
17. Octreotide. Lexi-Drugs. Hudson, OH: Lexicomp; 2022. online.lexi.com. Updated September 21, 2022. Accessed September 29, 2022.
18. Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association forthe Study of Liver Diseases. Hepatology. 2017;65(1):310-335.
19. Stanley AJ, Laine L. Management of acute upper gastrointestinal bleeding. BMJ. 2019;364:I536.
20. Fujushiro M, Iguchi M, Kakushima N, et al. Guidelines for endoscopic management of non-variceal upper gastrointestinal bleeding. Dig Endosc. 2016;28(4):363-378.
21. Sucralfate. Lexi-Drugs. Hudson, OH: Lexicomp; 2022. online.lexi.com. Updated September 24, 2022. Accessed September 29, 2022.
22. Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2017;65(1):310-335.
23. Octreotide. Lexi-Drugs. Hudson, OH: Lexicomp; 2022. online.lexi.com. Updated September 21, 2022. Accessed September 29, 2022.
24. Pemberton JH. Colonic diverticulosis and diverticular disease: epidemiology, risk factors, and pathogenesis. UptoDate. 2022. www.uptodate.com. Accessed September 29, 2022.
25. Saltzman JR. Angiodysplasia of the gastrointestinal tract. UptoDate. 2022. www.uptodate.com. Accessed September 29, 2022.
26. Brandt LJ, Feuerstadt P, Longstreth GF, Boley SJ. ACG clinical guideline: epidemiology, risk factors, patterns of presentation, diagnosis, and management of colon ischemia. Am J Gastroenterol. 2015;110(1):18-44.
27. Barkun AN, Douketis J, Noseworthy PA, et al. Management of patients on anticoagulants and antiplatelets during acute gastrointestinal bleeding and the peri-endoscopic period: a clinical practice guideline dissemination tool. Am J Gastroenterol. 2022;117(4):513-519.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






