US Pharm. 2020;45(11)(Specialty&Oncology suppl):3-8.
ABSTRACT: Epithelial ovarian cancer (OC), a common gynecologic malignancy, continues to be a leading cause of death in women, with just under 50% of patients surviving 5 years after diagnosis. Survival is often tied to the tumor stage; however, many women are diagnosed with advanced disease because of the asymptomatic nature of earlier stages. To date, surgery and systemic chemotherapy with platinum-based agents remain the standards of care. Recently, poly (adenosine diphosphate-ribose) polymerase inhibitors have demonstrated a significant benefit for patients diagnosed with OC. Accordingly, it is critical for the pharmacist to be familiar with the OC treatment armamentarium in order to ensure an optimal outcome for the patient.
Epithelial ovarian cancer (OC), the most common ovarian neoplasm, is the leading cause of death from gynecologic malignancies in the United States, as fewer than 40% of patients are cured.1,2 It was estimated that 21,750 new cases would be diagnosed and 13,940 OC-related deaths would occur in 2020. In the absence of treatment, the estimated 5-year survival rate is approximately 48.6%.3 The incidence of OC is known to increase with age, with a median age of 63 years at diagnosis. More than 70% of patients present with advanced disease; the location of the ovaries and the biology of most epithelial cancers make it difficult to diagnose OC in earlier stages.2-4 Factors associated with an increased risk of developing OC include nulliparity and older age (>35 years) at first pregnancy and first childbirth.5 Family history, including linkage to breast cancer genes 1 and 2 (BRCA1 and BRCA2) genotypes, is associated with early-onset disease.6 Currently, routine screening for early detection of OC is not standard and not recommended by cancer societies.
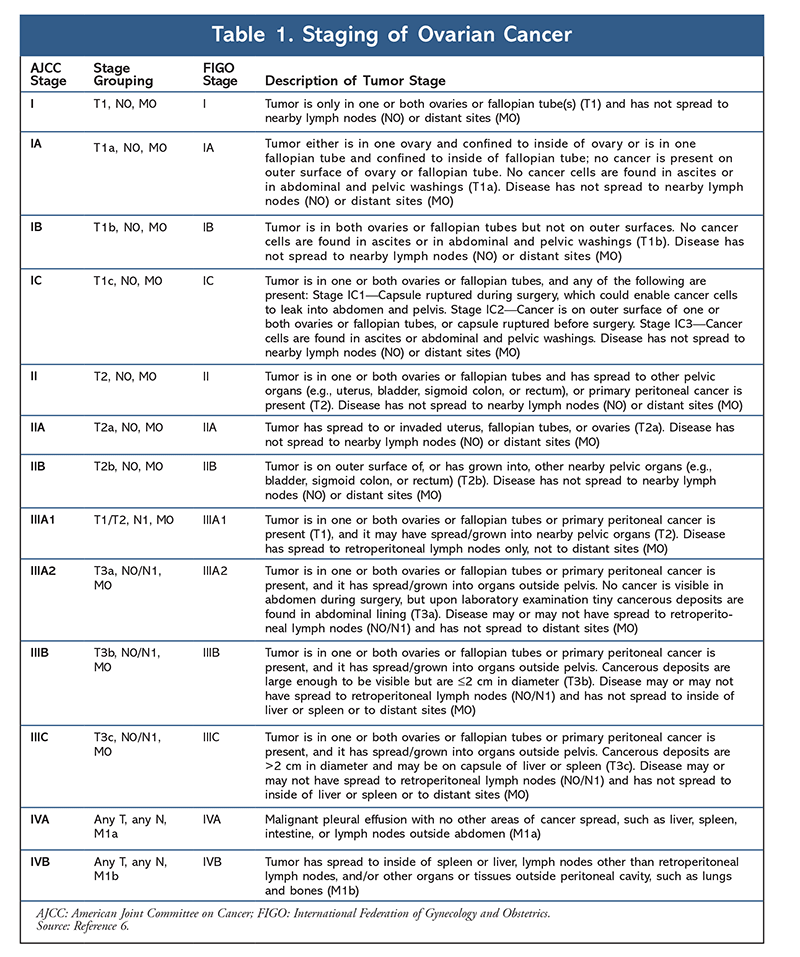
OC is staged according to the FIGO (International Federation of Gynecology and Obstetrics) system and the AJCC (American Joint Committee on Cancer) TNM (tumor, node, metastasis) system (TABLE 1).6 Determining the disease stage is an important element of the OC workup, as it will help guide treatment recommendations. OC is associated with several signs and symptoms, the most common of which are bloating, pelvic or abdominal pain, trouble eating or feeling full quickly, and urinary symptoms such as urgency or frequency. Other possible symptoms of OC include fatigue, upset stomach, constipation, back pain, painful intercourse, and menstrual changes. Women are more likely to have symptoms if the disease has spread, but even early-stage OC can cause symptoms.
Traditional Treatment Modalities
A primary treatment modality for OC is a multimodal regimen of debulking surgery and cytotoxic chemotherapy (ChT). Surgical procedures for debulking the tumor include total abdominal hysterectomy and unilateral or bilateral salpingo-oophorectomy (used if fertility preservation is preferred).6 The role of neoadjuvant ChT in OC remains controversial; however, the National Comprehensive Cancer Network (NCCN) suggests that cytotoxic ChT may be considered in patients with bulky stage III or IV disease that is unlikely to be completely resected via surgery alone or in patients who are poor surgical candidates.6 It is recommended that patients with stage II, III, or IV OC receive adjuvant ChT. Platinum plus taxane-based combination ChT is the standard of care for adjunctive ChT regardless of histologic subtype. Observation following debulking surgery is recommended for stage IA/IB OC, as survival rates exceeding 90% without adjunctive ChT have been demonstrated.7
For stage IV disease, the NCCN recommends treatment with platinum-based doublets, such as paclitaxel/carboplatin or docetaxel/carboplatin.6 Platinum plus taxane-based ChT has been the standard-of-care chemotherapeutic regimen since the 1990s. The combination of cisplatin plus paclitaxel administered as a 24-hour infusion was a first-line treatment based on a landmark trial demonstrating response rates of 59% in patients with advanced disease.7 Subsequent randomized trials using carboplatin plus paclitaxel as a 3-hour infusion demonstrated noninferior response rates, improved quality of life, and increased ease of administration compared with cisplatin/paclitaxel.8 Notably, carboplatin treatment was associated with lower rates of leukopenia, gastrointestinal (GI) disturbances, nephrotoxicity, and electrolyte imbalances compared with cisplatin but was associated with a higher incidence of thrombocytopenia. Given its noninferiority in patient outcomes and its superior safety profile, carboplatin is the preferred platinum agent for OC management.6 Because of their frailty, patients with poor performance status, comorbidities, stage IV disease, or advanced age may be appropriate candidates for single-agent, platinum-based ChT.
Both docetaxel and paclitaxel have been evaluated for combination therapy including a platinum for advanced-stage OC. A phase III trial comparing docetaxel/carboplatin versus paclitaxel/carboplatin for first-line treatment in stage III or IV OC after surgery demonstrated no difference in 2-year survival rates, with a near-28% complete response in both arms.9 However, higher rates of grade 3 to 4 neutropenia (including febrile neutropenia), GI disturbances, peripheral edema, and allergic reactions were observed with docetaxel, compared to higher rates of neuropathy and alopecia in the paclitaxel arm.9 Paclitaxel infused over 3 hours remains the preferred taxane for up-front treatment; however, preferential use of docetaxel may be considered in patients with preexisting neuropathy.6
Alternative Front-Line Options: Another front-line treatment option is the combination of pegylated doxorubicin and carboplatin.6 This regimen is supported by the MITO-2 phase III, randomized, controlled trial, which demonstrated noninferiority with carboplatin plus pegylated liposomal doxorubicin versus carboplatin plus paclitaxel.10 Liposomal doxorubicin offers a reduced toxicity profile and has the proposed benefit of concentrating within the tumor.11 Although higher rates of myelosuppression are observed with liposomal doxorubicin, rates of alopecia and neuropathy are significantly lower compared with paclitaxel/carboplatin. An emerging alternative that offers improved progression-free survival (PFS) rates when combined with ChT (14.1 months vs. 10.3 months, P <.001) is the antiangiogenic agent bevacizumab.12 The benefit is largely obtained in patients who continue bevacizumab as maintenance therapy after the traditional six cycles of carboplatin plus paclitaxel-based ChT.6 An option for maintenance therapy in patients who do not receive bevacizumab is continued use of paclitaxel for 12 cycles to mitigate the risk of recurrence.6
Intraperitoneal Therapy: Intraperitoneal therapy (IPT) is commonly reserved for stage III disease. IPT has been shown to increase survival by up to 16 months.6,7 This strategy employs localized ChT administration into the peritoneal cavity to improve targeted delivery. The combination of cisplatin and paclitaxel has been widely studied in this setting, demonstrating improved overall survival (OS) and PFS compared with standard IV ChT.13,14 However, the risk of adverse events (AEs) is reported to be much higher with IPT than with IV; therefore, patient-specific factors—including performance status and tolerability—should be considered prior to IPT initiation.
Progressive or Recurrent Disease: Despite effective front-line therapy, many patients will progress or have recurrent disease. The rate of relapse after front-line ChT for OC is as high as 62%, extending to 80% to 85% in stage III or IV disease. The onset of disease recurrence has important implications for treatment options in the relapsed setting. Poor prognosis has been associated with progression after two consecutive ChT regimens without sustaining a clinical benefit (platinum-refractory) and/or recurrence in less than 6 months (platinum-resistant).8 The estimated survival rate in platinum-sensitive patients is 12 to 24 months.15 Platinum-resistant disease portends a dismal prognosis, with survival of only 6 to 12 months.15 To date, no single therapeutic agent is the treatment of choice for recurrent disease. Generally, retreatment with a platinum agent is not recommended for platinum-resistant disease; however, altering the schedule of paclitaxel may produce a secondary response in selected patients.9,10 For patients with platinum-resistant disease, enrollment in a clinical trial is highly encouraged. Therapy selection is often based on provider experience and tailored to individual patient factors including medical history, comorbidities, end-organ function, access to care, and ability to adhere to therapy.
Recent Advances in Treatment: PARPis
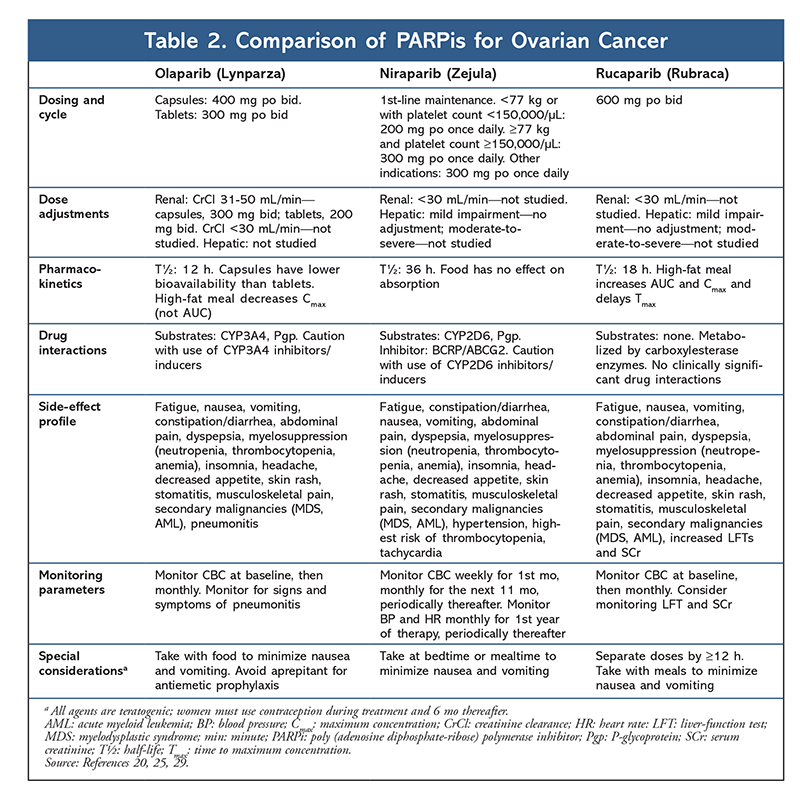
In the era of precision medicine, recent discoveries concerning the biological features of OC have paved the way for new targeted therapies. The identification of novel biomarkers/mutations—BRCA and homologous recombination deficiency (HRD)—that contribute to the pathogenesis of OC prompted the development of a new drug class, the poly (adenosine diphosphate [ADP]-ribose) polymerase inhibitors (PARPis). Given the rapid incorporation of three PARPis—olaparib, niraparib, and rucaparib—into the OC treatment landscape, it is critical for pharmacists to understand how these agents differ with regard to indication, line of therapy, BRCA/HRD mutation status requirements, dosing, toxicities, and drug interactions in order to ensure that patients obtain the greatest benefit from these therapies (TABLE 2).
Mechanism of Action: PARP plays an important role in DNA-repair pathways. Once a single-strand break (SSB) occurs in DNA, PARP enzymes restore genomic integrity through base excision repair and transfer of ADP moieties to acceptor proteins, a process known as PARylation. In the event that an SSB progresses to a double-strand break (DSB), homologous recombination takes place for the purpose of restoring normal cell function.16 Necessary elements in homologous recombination are the wild-type BRCA1 and BRCA2 genes. If a BRCA deficiency or HRD is present, homologous recombination will be ineffective at repairing DNA, resulting in irreversible DNA damage and cell death. Elucidation of this mechanism led to the therapeutic rationale for PARPi use in patients who harbor mutations in BRCA and HRD. Defective homologous recombination caused by dual BRCA deficiency and PARPi use results in DSBs, chromosomal instability, and ultimately apoptosis, a process known as synthetic lethality.16
Role in Treatment: Most patients with advanced OC have disease recurrence or progression after first-line platinum-based therapy. PARPis constitute the first oral, self-administered outpatient treatment option that can obviate the need for additional cytotoxic ChT. Olaparib and niraparib are approved as maintenance therapy for patients with newly diagnosed advanced OC after achieving a complete or partial response to initial platinum-based ChT or for patients with a baseline BRCA mutation. The landmark SOLO1 trial evaluated olaparib use in patients with a confirmed BRCA mutation.17 Compared with placebo, olaparib conferred a 70% lower risk of disease progression and better PFS at 36 months (60% vs. 27% for placebo; hazard ratio [HR], 0.3; P <.001).17 The PRIMA trial, which assessed the use of niraparib for maintenance therapy, did not require patients to have a baseline BRCA mutation.18 Patients enrolled in PRIMA were deemed to be at high risk for relapse based on visible residual disease after debulking surgery and ChT. Niraparib treatment resulted in a median PFS that was 5.6 months longer than that for the placebo group (HR, 0.62; P <.001), and median PFS was 11.5 months longer in patients with HRD (HR, 0.43; P <.001).18
All three PARPis carry an indication for maintenance therapy following two or more lines of platinum-based ChT with demonstration of treatment response regardless of BRCA-mutation status. Two trials, SOLO2 and Study 19, investigated olaparib in this setting.19,20 In Study 19, olaparib 400 mg administered twice daily improved PFS compared with placebo (8.4 months vs. 4.8 months; HR, 0.35; 95% CI, 0.24-0.49; P <.001), but no difference in OS was observed.20 Subsequently, in the SOLO2 trial, olaparib 300 mg twice daily demonstrated a 12.6-month improvement in PFS versus placebo (median 19.1 months vs. 5.5 months; HR, 0.30; P <.0001).19 The ARIEL3 trial led to approval of rucaparib based on a median 5.4-month improvement in PFS compared with placebo (P <.0001).21 Most recently, results of the NOVA trial on niraparib use mirrored previous studies demonstrating improved PFS (median 5.4 months; P <.001) versus placebo.22
Important findings from Study 19, ARIEL3, and NOVA were the notable improvement in PFS and the overall clinical benefit in patients with HRD or BRCA mutations versus nonmutated patients. These findings led to an opportunity for patients with a BRCA mutation and/or HRD to receive PARPi following ChT. All PARPis gained approval in this setting, although there are differences with regard to mutation-status requirement and the number of previous lines of therapy for each agent. Study 42 revealed that olaparib could be used as maintenance therapy after three lines of ChT in patients with BRCA mutations in whom further platinum therapy was unsuitable.23 Despite having significantly advanced disease, patients had a 34% overall response rate (ORR) and a median PFS of 7.9 months (95% CI, 5.6-9.6). Rucaparib’s approval for patients with BRCA mutation who previously received two lines of ChT is based on the ARIEL2 trial, which showed significantly longer PFS (12.8 months) in these patients versus 5.7 months and 5.2 months, respectively, in two BRCA wild-type subgroups (HR, 0.27; P <.001).24 In contrast to previous indications for BRCA mutations, niraparib most recently gained approval for patients with HRD who have undergone three or more lines of ChT.25 The QUADRA trial demonstrated that niraparib achieved a median ORR of 27.5% with HRD-positive disease, and the duration of response was prolonged (9.2 months).26
Not only have PARPis been integrated into OC treatment as monotherapy, but PARPi combinations with bevacizumab have emerged as a promising treatment option. The combination of olaparib plus bevacizumab versus bevacizumab monotherapy for maintenance therapy after first-line ChT was studied in the PAOLA-1 trial, and results showed a statistically significant improvement in PFS for combination therapy (median 22.1 months vs. 16.6 months; HR, 0.59; P <.0001).27 In AVANOVA2, which compared niraparib plus bevacizumab with niraparib alone for platinum-sensitive recurrent OC, niraparib plus bevacizumab significantly improved PFS compared with niraparib alone, with a median PFS of 11.9 months versus 5.5 months (HR, 0.35; CI, 0.21-0.57; P <.001).28 The decision to pursue combination therapy is patient-specific, and the potential benefits of bevacizumab should be weighed against the potential harms (e.g., hypertension, bleeding, thrombosis, poor wound healing).
Although PARPis are typically well tolerated, they are not without important AEs. AEs of all three PARPis include nausea, fatigue, and hematologic effects (anemia, thrombocytopenia, neutropenia).20,25,29 The incidence of hematologic toxicities is highest with niraparib, and these toxicities constitute the most common reason for dose modification or discontinuation.25 Interestingly, new evidence suggests that patients with a body weight of 77 kg or less or a platelet count of 150,000 cells per mL or less have a greater predisposition to developing thrombocytopenia, and patients meeting these criteria may be considered for preemptive dose adjustments.30 Secondary malignancies, albeit rare (0.5%-1%), have been observed with PARPis, and this association must be considered in the context of previous exposure to cytotoxic ChT that carries a known risk.20,25,29 PARPis are teratogenic; therefore, given that the occurrence of OC is higher in reproductive-age women, patients must be counseled on the use of birth control during and 6 months after PARPi treatment. Drug-specific toxicities include pneumonitis (olaparib), dysgeusia, increased cholesterol, and elevated liver enzymes.20,25,29
As PARPis continue to emerge in various settings in the treatment armamentarium for OC, pharmacists should understand the practical considerations surrounding their use. Olaparib is a major CYP3A4 substrate requiring dose adjustments with interacting medications.20 Rucaparib weakly inhibits CYP3A4, and niraparib is not a substrate or an inhibitor; therefore, these therapies are less susceptible to drug-drug interactions.25,29 Olaparib and rucaparib are dosed twice daily, whereas niraparib is dosed once daily. Olaparib comes in two formulations: a tablet and a capsule. The tablet is used more frequently in clinical practice given the higher bioavailability and reduced pill burden, that is, two 150-mg tablets twice daily compared with four 50-mg capsules twice daily.20,31 Coadministration with food does not impact bioavailability, and patients should be encouraged to take PARPis with a meal to alleviate nausea. Olaparib dosing requires careful consideration with regard to organ function, as dose adjustments are needed for both renal and hepatic impairment.
The Pharmacist’s Role
The pharmacist is a vital member of the healthcare team for OC management, including patient education and the selection, dosing, and monitoring of ChT. The pharmacist should be aware of pertinent dosing and monitoring parameters for front-line platinum and taxane-based regimens. Paclitaxel, like most ChT, is dosed based on body-surface area, whereas carboplatin is dosed to target an AUC calculated using the Calvert formula.32 Carboplatin lacks the renal and ototoxic effects of cisplatin, but it carries a significant risk of thrombocytopenia that warrants a routine CBC prior to each cycle.6 Paclitaxel is associated with neuropathy and alopecia based on its disruption of microtubule synthesis. Routine screening and reassessment for neuropathy should be performed, and appropriate guideline-directed management should be initiated if symptoms emerge.6 Hypertension is a hallmark AE of bevacizumab; blood-pressure screenings prior to initiation and during treatment are required, and it is recommended to withhold therapy when systolic blood pressure is 160 mmHg or higher.6 Proteinuria can occur with long-term bevacizumab therapy; therefore, urinalysis with screening of urine protein-to-creatinine ratios to assess for proteinuria should be performed routinely to ensure therapy safety.
Taxanes and platinum-based agents are the most common sources of hypersensitivity reactions.33 Monitoring and management of hypersensitivity reactions are critical in OC patients given the important role of these front-line agents. Determining the cause of hypersensitivity in a patient receiving multiple chemotherapeutic agents can be challenging; however, the timing of onset may give some insight into the potential causative agent. Paclitaxel-induced hypersensitivity typically occurs within the first 10 to 15 minutes of infusion and is associated with bronchospasm, hypotension, and rash. Paclitaxel hypersensitivity may be due to the drug compound itself or to the cremophor vehicle used in the formulation.33 Premedication is required with the use of H1 and H2 antagonists and corticosteroids. Carboplatin-induced hypersensitivity is a delayed phenomenon, often occurring around the sixth or seventh treatment cycle, and is characterized by a erythematous rash, urticaria, and bronchospasm. In patients who develop this reaction, rechallenging with carboplatin is not recommended; transitioning to an alternative regimen is preferred.33 Desensitization protocols for carboplatin exist; however, allergy specialists should be consulted to ensure that the patient is in an acute-care monitored setting to facilitate safe desensitization.34 For PARPis, adherence to therapy is essential and can be optimized by counseling patients about the high potential for nausea and fatigue. Pharmacists should counsel patients to take the medication after a meal and should identify patients who may benefit from prophylactic antiemetic use. In anticipation of liberal antiemetic use, prevention of drug interactions with olaparib and aprepitant—a strong CYP3A4 inhibitor—is essential. The pharmacist plays an important role in facilitating the selection and subsequent monitoring of traditional and novel therapies for patients with OC.
REFERENCES
1. Prat J. New insights into ovarian cancer pathology. Ann Oncol. 2012;23(suppl 10):x111-x117.
2. Jelovac D, Armstrong DK. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J Clin. 2011;61:183-203.
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30.
4. Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review, 1975-2014. Bethesda, MD: National Cancer Institute; 2017.
5. Fleming GF, Seidman J, Lengyel E. Epithelial ovarian cancer. In: Barakat RR, Markman M, Randall ME, eds. Principles and Practice of Gynecologic Oncology. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2013:757-847.
6. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Ovarian cancer. Version 1.2020. www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Accessed October 6, 2020.
7. Winter-Roach BA, Kitchener HC, Dickinson HO. Adjuvant (post-surgery) chemotherapy for early stage epithelial ovarian cancer. Cochrane Database Syst Rev. 2009;(3):CD004706.
8. Ozols RF, Bundy BN, Greer BE, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194-3200.
9. Vasey PA, Jayson GC, Gordon A, et al. Phase III randomized trial of docetaxel-carboplatin versus paclitaxel-carboplatin as first-line chemotherapy for ovarian carcinoma. J Natl Cancer Inst. 2004;96:1682-1691.
10. Pignata S, Scambia G, Ferrandina G, et al. Carboplatin plus paclitaxel versus carboplatin plus pegylated liposomal doxorubicin as first-line treatment for patients with ovarian cancer: the MITO-2 randomized phase III trial. J Clin Oncol. 2011;29:3628-3635.
11. Gibson JM, Alzghari S, Ahn C, et al. The role of pegylated liposomal doxorubicin in ovarian cancer: a meta-analysis of randomized clinical trials. Oncologist. 2013;18:1022-1031.
12. Burger RA, Brady MF, Rhee J, et al. Independent radiologic review of the Gynecologic Oncology Group Study 0218, a phase III trial of bevacizumab in the primary treatment of advanced epithelial ovarian, primary peritoneal, or fallopian tube cancer. Gynecol Oncol. 2013;131:21-26.
13. Markman M, Bundy BN, Alberts DS, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:1001-1007.
14. Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34-43.
15. Matsuo K, Lin YG, Roman LD, Sood AK. Overcoming platinum resistance in ovarian carcinoma. Expert Opin Investig Drugs. 2010;19:1339-1354.
16. Franzese E, Centonze S, Diana A, et al. PARP inhibitors in ovarian cancer. Cancer Treat Rev. 2019;73:1-9.
17. Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379(26):2495-2505.
18. González-Martín A, Pothuri B, Vergote I, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381:2391-2402.
19. Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274-1284.
20. Lynparza (olaparib) package insert. Missisauga, ON: AstraZeneca Canada Inc; May 2020.
21. Coleman RL, Oza AM, Lorusso D, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949-1961.
22. del Campo JM, Matulonis UA, Malaner S, et al. Niraparib maintenance therapy in patients with recurrent ovarian cancer after a partial response to the last platinum-based chemotherapy in the ENGOT-OV16/NOVA trial. J Clin Oncol. 2019;37:2968-2973.
23. Domchek SM, Aghajanian C, Shapira-Frommer R, et al. Efficacy and safety of olaparib monotherapy in germline BRCA1/2 mutation carriers with advanced ovarian cancer and three or more lines of prior therapy. Gynecol Oncol. 2016;140:199-203.
24. Swisher EM, Lin KK, Oza AM, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017;18:75-87.
25. Zejula (niraparib) package insert. Research Triangle Park, NC: GlaxoSmithKline; April 2020.
26. Moore KN, Secord AA, Geller MA, et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20:636-648.
27. Ray-Coquard I, Pautier P, Pignata S, et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381:2416-2428.
28. Mirza MR, Åvall Lundqvist E, Birrer MJ, et al. Niraparib plus bevacizumab versus niraparib alone for platinum-sensitive recurrent ovarian cancer (NSGO-AVANOVA2/ENGOT-ov24): a randomised, phase 2, superiority trial. Lancet Oncol. 2019;20:1409-1419.
29. Rubraca (rucaparib) package insert. Boulder, CO: Clovis Oncology, Inc; May 2020.
30. Berek JS, Matulonis UA, Peen U, et al. Safety and dose modification for patients receiving niraparib. Ann Oncol. 2018;29:1784-1792.
31. Lynparza (olaparib) package insert. Wilmington, DE: AstraZeneca Pharmaceuticals LP; September 2018.
32. Mazumdar M, Smith A, Tong WP, Motzer RJ. Calvert’s formula for dosing carboplatin: overview and concerns of applicability in high-dose setting. J Natl Cancer Inst. 2000;92:1434-1436.
33. Boulanger J, Boursiquot JN, Cournoyer G, et al. Management of hypersensitivity to platinum- and taxane-based chemotherapy: CEPO review and clinical recommendations. Curr Oncol. 2014;21(4):e630-e641.
34. Takase N, Matsumoto K, Onoe T, et al. 4-Step 4-h carboplatin desensitization protocol for patients with gynecological malignancies showing platinum hypersensitivity: a retrospective study. Int J Clin Oncol. 2015;20:566-573.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





