US Pharm. 2010;35(9):HS-2-HS-8.
Pregnancy is associated with increased levels of emotional and physical stress. Women with preexisting conditions such as hypertension and diabetes require intense prenatal monitoring by health care professionals. Pharmacists in direct contact with patients can play an integral role in identifying signs and symptoms that require immediate care. Two conditions that require emergent treatment in pregnant women are severe preeclampsia and diabetic ketoacidosis.
SEVERE PREECLAMPSIA
Hypertensive disorders can affect 6% to 8% of women and increase the risk of morbidity and mortality in both the expectant mother and the unborn child.1,2 Hypertension in pregnancy is divided into four categories: chronic hypertension, gestational hypertension, preeclampsia, and preeclampsia superimposed on chronic hypertension. The focus in this article is on severe preeclampsia, but a brief discussion of preeclampsia is warranted.
Preeclampsia, a pregnancy-specific syndrome of unknown etiology, is a multiorgan disease process characterized by the development of hypertension and proteinuria after 20 weeks' gestation.1,2 See TABLE 1 for diagnostic criteria.1,2 History of antiphospholipid antibody syndrome, chronic hypertension, chronic renal disease, elevated body-mass index, age 40 years or older, multiple gestation, nulliparity, preeclampsia in a previous pregnancy, and pregestational diabetes mellitus increase a woman's risk of preeclampsia.1
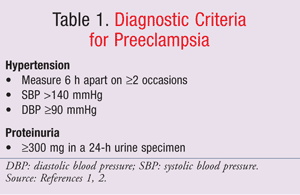
Preeclampsia is classified as mild or severe based on the degree of hypertension, the level of proteinuria, and the presence of symptoms resulting from the involvement of the kidneys, brain, liver, and cardiovascular system. The incidence of severe preeclampsia is 0.9% in the United States.3 Severe preeclampsia is associated with multiorgan involvement such as pulmonary edema, seizures, oliguria, thrombocytopenia (platelet count <100,000/mm3), abnormal liver enzymes in association with epigastric or right upper quadrant pain, and central nervous system (CNS) involvement (altered mental status, headaches, blurred vision, blindness).2
The timing of the diagnosis affects the rate of neonatal complications. Women who develop severe preeclampsia in their second trimester are at increased risk for neonatal complications compared with women who develop it after 35 weeks' gestation.3,4
Severe preeclampsia requires emergent management because it is associated with an increased risk of maternal mortality and morbidity such as convulsions, pulmonary edema, acute renal failure, liver failure, disseminated intravascular coagulopathy, and stroke.2
Women who develop severe preeclampsia are usually admitted to the hospital, limited to bed rest, and monitored closely for signs and symptoms of seizures. The goals of treatment are to prevent seizures, lower blood pressure to avoid maternal end-organ damage, and expedite delivery.1 Although delivery is the only cure for preeclampsia, the decision to deliver depends on maternal and fetal factors such as gestational age, lung maturity, and signs of fetal compromise.
Women who develop severe preeclampsia prior to 23 weeks' gestation should be offered pregnancy termination because expectant management results in high maternal and perinatal morbidity and mortality.3-5 Patients who develop severe preeclampsia after 34 weeks' gestation or who show signs of fetal lung maturity should deliver.2,3 Patients who do not achieve blood pressure control or who show signs of maternal or fetal deterioration should be delivered within 24 hours, irrespective of other factors. For patients with severe preeclampsia at 24 to 34 weeks' gestation, current opinion is to either intervene (delivery or pregnancy termination) or expectantly manage existing symptoms. Odendaal et al and Sibai et al found improved perinatal outcomes with expectant management in selected patients with severe preeclampsia at 28 to 34 weeks' gestation.3
HELLP Syndrome
Hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome develops in approximately 20% of women with severe preeclampsia.5 The presence of HELLP syndrome increases the risk of adverse outcomes such as pulmonary edema, acute renal failure, disseminated vascular coagulopathy, abruptio placentae, hepatic hemorrhage or failure, and fetal or maternal death.6 In HELLP syndrome, women present with nonspecific complaints of right upper quadrant or epigastric pain, nausea, and vomiting.1,6
Limited information about treatment options is available, as most studies evaluating treatment exclude patients with HELLP syndrome. Most experts recommend prompt vaginal delivery within 48 hours, as cesarean delivery can increase the risk of bleeding because of low platelet count and reduced intravascular volume, which will adversely affect blood pressure.1,3 Visser and Wallenberg and van Runnard Heimel et al offer a contrary opinion, recommending expectant treatment in selected patients with severe preeclampsia and HELLP syndrome. Although these findings prolonged pregnancy, perinatal outcomes were not improved.3
Pharmacologic Management of Severe Preeclampsia
Medications used to treat preeclampsia are presented in TABLE 2.1,3,7
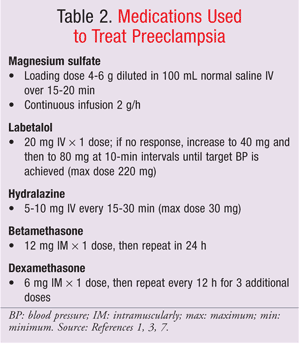
Magnesium sulfate is the mainstay of therapy, as it prevents seizures by slowing neuromuscular conduction and depressing CNS irritability without affecting blood pressure.1 IV labetalol and hydralazine are commonly used to achieve blood pressure control.3,7 Patients should be closely monitored for blood pressure, urine output, cerebral status, and the presence of epigastric pain, tenderness, labor, or vaginal bleeding. Fetal monitoring parameters include heart rate, evidence of lung maturity, amniotic-fluid volume, and signs of fetal compromise.1,2 Patients who do not respond to pharmacologic management and who show signs and symptoms of deterioration should deliver within 24 to 48 hours, irrespective of gestational age.1,2,7
Corticosteroids such as betamethasone and dexamethasone can accelerate fetal lung maturity. The use of corticosteroids may reduce the risk of neonatal respiratory distress syndrome, intravascular hemorrhage, infection, and death.1,2 Interventionist management involves induction or cesarean delivery after 12 to 24 hours of corticosteroid treatment. Expectant management involves monitoring both mother and fetus closely and delaying delivery, when possible, to reduce neonatal complications (TABLE 2).1,2
Most patients improve after delivery and should be monitored for eclampsia for the next 48 hours. Patients should continue taking magnesium sulfate for 12 to 24 hours.1 Blood pressure, fluid intake, and urine output should be closely monitored.
DIABETIC KETOACIDOSIS
Diabetic ketoacidosis (DKA) during pregnancy is a condition that necessitates emergent attention because of the significant negative consequences to the mother and the developing baby. Although DKA in pregnancy is uncommon (incidence of 1% to 9% in pregnancies already complicated by diabetes), clinicians must be ready to initiate aggressive therapy.8,9 Similar to management in nonpregnant patients, prevention of ketoacidosis is the primary goal in diabetic pregnant women.
Diabetes first diagnosed during any trimester of pregnancy is classified as gestational diabetes. Ketoacidosis can occur in pregnancies complicated by type 1, type 2, or gestational diabetes. Although type 1 pregnancy ketoacidosis is most common, patients with type 2 or gestational diabetes should be monitored for DKA throughout pregnancy.10
Changes during pregnancy, such as increased insulin resistance, dehydration secondary to emesis, and stress, predispose the pregnant diabetic patient to DKA. Ketoacidosis most often presents during the second or third trimester, when insulin resistance is at its peak. Several common precipitating factors include acute illness or infection, insulin-pump failure, noncompliance with the prescribed insulin regimen, and medication-induced ketoacidosis due to steroid or beta-adrenergic agonist use.9
Presenting symptoms of ketoacidosis in pregnant women are generally the same as in nonpregnant patients, but with aggressive onset. Symptoms include nausea, vomiting, polyuria, polydypsia, and changes in mental status. Laboratory findings may include elevated anion gap, acidemia, hyperglycemia, ketonemia, or renal dysfunction.8,10 DKA in pregnancy may occur with blood glucose levels less than 200 mg/dL, in which case the only presenting symptoms may be nausea, vomiting, and decreased caloric intake.9 See SIDEBAR 1 for a case report.11

Because nausea, vomiting, and increased urinary frequency are common during pregnancy, women may minimize the significance of these symptoms and delay care. Urine or blood ketone testing is recommended for pregnant women with diabetes who experience weight loss or are unable to maintain adequate oral intake owing to excessive nausea and vomiting.12 Health care professionals working with diabetic pregnant women should emphasize the need for immediate action if dietary patterns change.
Fetal effects of maternal DKA range from long-term cognitive deficits to fetal demise.9,10 Since the introduction of insulin, fetal loss from DKA has been significantly reduced; however, fetal mortality still occurs in 10% to 53% of pregnancies involving DKA.9
Maternal DKA produces fetal distress through several complex mechanisms occurring simultaneously. Fetal hypoxia results from maternal volume depletion and acidemia. The direct transfer of ketoacids across the placenta leads to fetal acidosis. Also, fetal hyperinsulinemia produces an increased oxygen demand in the fetus, worsening fetal distress. In addition, electrolyte imbalance--such as fetal hypokalemia--may place the fetus at risk for serious cardiac arrhythmia or arrest, and maternal hypophosphatemia leads to decreased oxygen delivery to the fetus.9,10
Pharmacologic Management of DKA
Management of DKA in pregnant patients follows the same pattern as in nonpregnant patients, with the addition of fetal monitoring. Primary goals of therapy for the mother include rehydration; correction of metabolic acidosis; correction of electrolyte disturbances; blood glucose control; and finding and treating the precipitating cause of the DKA. Effective management of maternal risk will reduce stress on the fetal environment and improve the chances of fetal preservation.8,9
Glucose targets for pregnant patients with diabetes are more aggressive than those for nonpregnant patients (TABLE 3).12,13 Therapeutic blood glucose goals may be achieved most rapidly by initiating a continuous insulin infusion with aggressive blood glucose monitoring and titration to goal. Once blood glucose is stable within target parameters and the patient is able to tolerate oral intake, the insulin infusion should be titrated off, with overlap of subcutaneous insulin initiation.
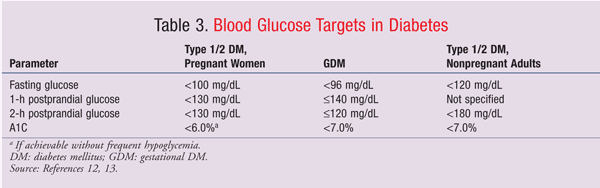
Critically ill patients receiving insulin infusion therapy should initially be treated to a glucose goal of 140 mg/dL to 180 mg/dL.14 Then, when the patient is stabilized, glucose targets follow those for pregnant patients with diabetes (TABLE 3). The Fifth International Workshop-Conference on Gestational Diabetes Mellitus recommends maintaining fasting glucose of <96 mg/dL, 1-hour postprandial glucose of £140 mg/dL, and 2-hour postprandial glucose of £120 mg/dL.12 The American Diabetes Association recommends that pregnant patients with type 1 or type 2 diabetes maintain a fasting glucose of <100 mg/dL and peak postprandial glucose of <130 mg/dL.13
Regular human insulin and Neutral Protein Hagedorn (NPH) insulin have been studied and utilized extensively in pregnancy, and have been found to be safe and effective.12 Evidence from retrospective studies shows that the long-acting insulin analogue glargine is safe and effective also; it may be superior to NPH, based on a lower incidence of hypoglycemia and improved fasting blood glucose levels.15-18 Although data from randomized, controlled trials are lacking for glargine use in pregnancy, clinicians are widely accepting glargine as an alternative to NPH, owing to increased patient satisfaction with a once-daily injection regimen. Aspart and lispro are also efficacious, safe options for short-acting insulin analogues, when necessary.19,20
CONCLUSION
Effective management of preeclampsia and DKA can improve maternal and fetal morbidity and mortality. Pharmacists in acute or community settings can help recognize and facilitate the prompt management of these serious conditions in their patients.
REFERENCES
2007;18:590-593.
1. Leeman L, Fontaine P. Hypertensive disorders of pregnancy. Am Fam Physician. 2008;78:93-100.
2. Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102:181-192.
3. Sibai BM, Barton JR. Expectant management of severe preeclampsia remote from term: patient selection, treatment, and delivery indications. Am J Obstet Gynecol. 2007;196:514e1-514e9.
4. Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105:402-410.
5. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002;99:159-167.
6. Sibai BM. Diagnosis, controversies, and management of the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Obstet Gynecol. 2004;103:981-991.
7. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1-S22.
8. Schneider MB, Umpierrez GE, Ramsey RD, et al. Pregnancy complicated by diabetic ketoacidosis: maternal and fetal outcomes. Diabetes Care. 2003;26:958-959.
9. Ramin KD. Diabetic ketoacidosis in pregnancy. Obstet Gynecol Clin North Am. 1999;26:481-488.
10. Kamalakannan D, Baskar V, Barton DM, Abdu TA. Diabetic ketoacidosis in pregnancy. Postgrad Med J. 2003;79:454-457.
11. Tarif N, Al Badr W. Euglycemic diabetic ketoacidosis in pregnancy. Saudi J Kidney Dis Transpl.
12. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30:S251-S260.
13. Standards of medical care in diabetes--2010. Diabetes Care. 2010;33:S11-S61.
14. Executive summary: standards of medical care in diabetes--2010. Diabetes Care. 2010;33:S4-S10.
15. Imbergamo MP, Amato MC, Sciortino G, et al. Use of glargine in pregnant women with Type 1 diabetes mellitus: a case-control study. Clin Ther. 2008;30:1476-1484.
16. Smith JG, Manuck TA, White J, Merrill DC. Insulin glargine versus neutral protamine Hagedorn insulin for treatment of diabetes in pregnancy. Am J Perinatol. 2009;26:57-62.
17. Fang YMV, Mackeen D, Egan JFX, Zelop CM. Insulin glargine compared with Neutral Protamine Hagedorn insulin in the treatment of pregnant diabetics. J Matern Fetal Neonatal Med. 2009;22:249-253.
18. Egerman RS, Ramsey RD, Kao LW, et al. Perinatal outcomes in pregnancies managed with antenatal insulin glargine. Am J Perinatol. 2009;26:591-595.
19. Hod M, Damm P, Kaaja R, et al. Fetal and perinatal outcomes in type 1 diabetes pregnancy: a randomized study comparing insulin aspart with human insulin in 322 subjects. Am J Obstet Gynecol. 2008;198:186.e1-186.e7.
20. Wyatt JW, Frias JL, Hoyme HE, et al. Congenital anomaly rate in offspring of mothers with diabetes treated with insulin lispro during pregnancy. Diabetic Med. 2004;22:803-807.
To comment on this article, contact rdavidson@uspharmacist.com.





