US Pharm. 2019;44(7):47-48.
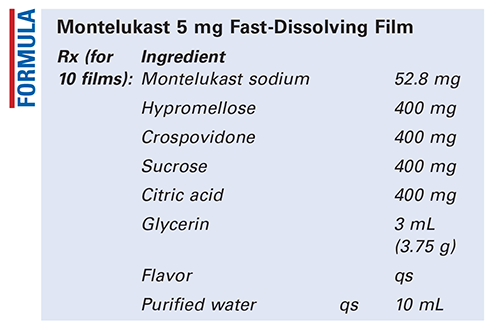
Method of Preparation: Calculate the quantity of each ingredient for the amount to be prepared. Accurately weigh or measure each ingredient. Levigate the hypromellose and crospovidone with the glycerin. Incorporate the montelukast sodium and mix well. Add the sucrose, citric acid, and flavor to about 4 mL of purified water. Combine the two mixtures and mix well. Add sufficient purified water to final volume and mix well. Cast the mixture onto a glass plate with boundaries situated to prevent the film from overflowing. Allow the preparation to dry in a hot air oven at 40°C for 24 hours. Cut or punch out the required size of film calculated to provide the desired dose (area/10 = one dose). Package and label.
Use: Montelukast sodium fast-dissolving films have been used in the treatment of asthma.1
Packaging: Package in tight, light-resistant containers.2
Labeling: Keep out of reach of children. Store in a cool place. Use only as directed. Discard after ____ [time period].
Stability: Check the current edition of the U.S. Pharmacopeia for the appropriate beyond-use date for this compounded preparation.2
Quality Control: Quality-control assessment can include weight, specific gravity, active drug assay, color, texture of surface, appearance, feel, melting test, dissolution test, physical observation, and physical stability.3
Discussion: Montelukast sodium (Singulair, C35H35ClNNaO3S, MW 608.17) occurs as a white or almost white, hygroscopic powder that is freely soluble in water and freely to very soluble in alcohol. One mg of montelukast is contained in 1.056 mg of montelukast sodium.2,4
Hypromellose (hydroxypropyl methylcellulose [HPMC], cellulose, hydroxypropyl methyl ether, Methocel, Pharmacoat) occurs as an odorless and tasteless, white or creamy-white, fibrous or granular powder. It is used as a coating agent, film-former, rate-controlling polymer, stabilizing agent, suspending agent, tablet binder, and viscosity-increasing agent. The pH of a 1% aqueous solution is in the range of 5.5 to 8.0. Hypromellose is soluble in cold water.5
Crospovidone [(C6H9NO)n where n is >1,000,000 Kollidon CL-M] is a water insoluble, synthetic, cross-linked homopolymer of N-vinyl-2-pyrrolidinone. It occurs as a white to creamy-white, finely divided, free-flowing, practically tasteless, odorless or nearly odorless, hygroscopic powder.6
Sucrose (beet sugar, cane sugar, refined sugar, saccharose) is obtained from sugar cane, sugar beet, or other sources. It occurs as colorless crystals, as crystalline masses or blocks, or as a white, crystalline powder that is odorless and has a sweet taste. Sucrose is soluble in water 1:0.5, alcohol 1:400, and 95% ethanol 1:170.7
Citric acid (citric acid monohydrate, C6H8O7.H2O) occurs as colorless or translucent crystals or as a white, crystalline, efflorescent powder that is odorless and has a strong, tart, acidic taste. One gram is soluble in less than 1 mL of water and in 1.5 mL of ethanol.8
Glycerin (glycerol, 1,2,3-propane triol) occurs as a clear, colorless, odorless, viscous, hygroscopic liquid with a sweet taste. It has a specific gravity of about 1.25 and a melting point of 17.8°C. Glycerin is miscible with water, methanol, and 95% ethanol.9
REFERENCES
1. Ghorwade V, Patil A, Hullale A. Fast dissolving films: a novel approach for the delivery of montelukast sodium. Int J Pharm Pharmaceutical Sci. 2012;4:228-232.
2. U.S. Pharmacopeia/National Formulary [current revision]. Rockville, MD: U.S. Pharmacopeial Convention, Inc; June 2019.
3. Allen LV Jr. Standard operating procedure for performing physical quality assessment of suppositories, troches, lollipops and sticks. IJPC. 1999;3:56-57.
4. Allen LV Jr. Remington: The Science and Practice of Pharmacy. 22nd ed. London, England: Pharmaceutical Press; 2013:1606-1607.
5. Rogers TL, Penz FK, Zelenik JA. Hypromellose. In: Sheskey PJ, Cook WG, Cable CT, eds. Handbook of Pharmaceutical Excipients. 8th ed. London, England: Pharmaceutical Press; 2017:468-472.
6. Kibbe AH. Crospovidone. In: Sheskey PJ, Cook WG, Cable CT, eds. Handbook of Pharmaceutical Excipients. 8th ed. London, England: Pharmaceutical Press; 2017:285-287.
7. Shah HC, Singh KK. Sucrose. In: Sheskey PJ, Cook WG, Cable CT, eds. Handbook of Pharmaceutical Excipients. 8th ed. London, England: Pharmaceutical Press; 2017:938-942.
8. Amidon GE. Citric acid monohydrate. In: Sheskey PJ, Cook WG, Cable CT, eds. Handbook of Pharmaceutical Excipients. 8th ed. London, England: Pharmaceutical Press; 2017:250-253.
9. Alvarez-Nunez FA, Daurio D. Glycerin. In: Sheskey PJ, Cook WG, Cable CT, eds. Handbook of Pharmaceutical Excipients. 8th ed. London, England: Pharmaceutical Press; 2017:401-405.
To comment on this article, contact rdavidson@uspharmacist.com.





