US Pharm. 2021;46(4):46-48.
Obstructive sleep apnea (OSA) is characterized by an intermittent cessation of breathing that occurs when the upper airway collapses during sleep. This obstruction of the airway may result in reduced oxygenation in the body.1 Other conditions associated with OSA include inadequate sleep, tiredness, snoring, and impaired cognitive function.2-4 Risk factors for OSA include obesity, older age, male sex, structural abnormalities in the face or airway, and possibly smoking and alcohol. Untreated OSA may lead to an increased risk of diabetes, heart failure, arrhythmias, hypertension, and mortality.4
Epidemiology
Although OSA is more common in males than in females, the difference in prevalence between the sexes lessens with age. Men and women aged 30 to 49 years were reported to have moderate OSA prevalence rates of 10% and 3%, respectively, compared with men and women aged 50 to 70 years, who had prevalence rates of 17% and 9%, respectively.5 It is likely that OSA is underdiagnosed, and prevalence rates could be as high as 90% in the United States.5 Prevalence rates may vary depending on how different studies define a diagnosis of OSA in the study participants.6 Data support an increased risk of OSA in African Americans and Asians compared with Caucasians. A possible contributing factor to racial differences in prevalence could be dissimilarities in facial structure.5
Diagnosis
The American Academy of Sleep Medicine (AASM) provides diagnostic criteria for OSA in its most recent clinical practice guidelines.6 An OSA diagnosis requires a breathing test performed while the patient is asleep. Polysomnography (PSG), the first-line diagnostic tool for OSA, determines how many times per hour breathing is disrupted. These disruptions may be due to interrupted breathing or episodes of arousal caused by effortful breathing. If breathing is disrupted five or more times per hour and the patient experiences symptoms such as unrestful sleep, fatigue, snoring, and noticeable apneas, a diagnosis of OSA is appropriate. If the patient experiences 15 or more breathing disruptions, OSA can be diagnosed without the presence of any other symptoms. Questionnaires and home-testing methods are not recommended without the use of PSG.6 Diagnosis of the severity of OSA is based on the Apnea-Hypopnea Index (AHI) score, according to which a score of 15 or higher indicates moderate-to-severe OSA.2
Treatment
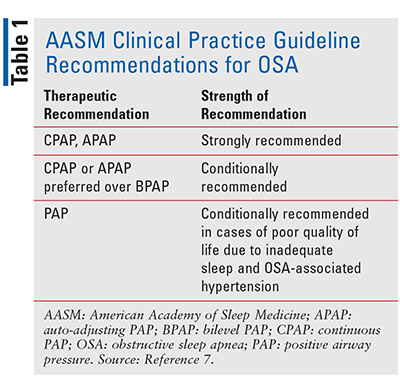
First-line treatment for OSA is the use of a positive airway pressure (PAP) machine. TABLE 1 summarizes the AASM’s therapeutic recommendations for OSA.7 In general, the two forms of PAP that are recommended in most patients who require OSA treatment are continuous PAP (CPAP) and auto-adjusting PAP (APAP). Bilevel PAP may be used, but it is considered an alternative therapy to CPAP and APAP. The AASM recommends that PAP treatment be initiated in patients with OSA who have daytime sleepiness. Because some OSA patients with hypertension may be less willing to use a PAP machine if they can control their blood pressure with medication and are not experiencing daytime sleepiness, the AASM considers the PAP recommendation for such patients to be conditional.7 Potential adverse effects of PAP treatment include dry mouth, sore throat, and sinus infections. Although PAP is first-line treatment, alternative therapies may be necessary in patients who are nonadherent to PAP.3
Inspire Upper Airway Stimulation System
The Inspire upper airway stimulation (UAS) system (FIGURE 1), which was developed by Inspire Medical Systems, Inc., was recently approved to treat moderate-to-severe OSA.8,9 This device is indicated for use in OSA patients aged 22 years and older who demonstrate failure of or intolerance to PAP treatment. An AHI score of 15 to 65 with PAP use is considered treatment failure. If the patient does not use a PAP machine for more than 4 hours per night or for more than 5 nights per week, he or she is considered to be intolerant to PAP treatment. Patients aged 18 to 21 years with an AHI score of 15 to 65 may use UAS if they meet the following criteria: They are intolerant to or have failed PAP therapy; do not have complete soft-palate collapse; do not qualify for or have failed adenotonsillectomy therapy; or have exhausted all other therapeutic options.10

The Inspire UAS system device is surgically implanted in the patient’s chest. After a recovery period of 1 month, the device may be activated. The device, which includes leads that can sense respiratory patterns in the body, stimulates the hypoglossal nerve to prevent obstruction.8,10 The patient can use the Inspire remote to turn the therapy on at bedtime and off upon waking.8
Efficacy and Adverse Effects
In an uncontrolled cohort study in which the Inspire UAS system was implanted in patients with moderate-to-severe OSA, there were significant improvements in patients’ AHI scores and oxygen desaturation indexes (ODIs).2 Approximately 78% of these patients were assessed in a different study 5 years later.11 This follow-up study results found that 48% of patients had an AHI score below 5 and that 78% had an AHI score below 15. Snoring was reported to have decreased from baseline in the initial study, and ODI scores, daytime functioning, and daytime-sleepiness symptoms were noted to have improved after device implantation. The device was relatively well tolerated at 60 months post implantation.11 Procedure-related adverse effects included incision-site and postoperative discomfort, tongue weakness, intubation effects, headache, other postoperative symptoms (e.g., nausea, vomiting, pain), and infection; device-related adverse effects included electrical stimulation–related discomfort, tongue abrasion, dry mouth, device-related pain, device-functionality problems, infection, and other adverse effects (e.g., headache, soreness, coughing, choking).11
Conclusion
The Inspire UAS system has the potential to benefit patients who cannot tolerate or fail to respond to PAP treatment. The device has demonstrated efficacy beyond the first year of implantation and has been shown to be well tolerated by patients. The Inspire UAS system is a convenient therapeutic option and could be considered as an alternative to PAP treatment for OSA in eligible patients. More information on this device is available on the FDA’s website.8
REFERENCES
1. Certal VF, Zaghi S, Riaz M, et al. Hypoglossal nerve stimulation in the treatment of obstructive sleep apnea: a systematic review and meta-analysis. Laryngoscope. 2015;125(5):1254-1264.
2. Strollo PJ Jr, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139-149.
3. Soose RJ, Gillespie MB. Upper airway stimulation therapy: a novel approach to managing obstructive sleep apnea. Laryngoscope. 2016;126(suppl 7):S5-S8.
4. Cowie MR. Sleep apnea: state of the art. Trends Cardiovasc Med. 2017;27(4):280-289.
5. Rundo JV. Obstructive sleep apnea basics. Cleve Clin J Med. 2019;86(9 suppl 1):2-9.
6. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13(3):479-504.
7. Patil SP, Ayappa IA, Caples SM, et al. Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2019;15(2):335-343.
8. FDA. Inspire® Upper Airway Stimulation—P130008/S039. www.fda.gov/medical-devices/recently-approved-devices/inspirer-upper-airway-stimulation-p130008s039. Accessed November 1, 2020.
9. Inspire Medical Systems, Inc. Inspire sleep apnea innovation. www.inspiresleep.com/. Accessed November 12, 2020.
10. FDA. Inspire upper airway stimulation system implant manual. www.accessdata.fda.gov/cdrh_docs/pdf13/P130008D.pdf. Accessed November 1, 2020.
11. Woodson BT, Strohl KP, Soose RJ, et al. Upper airway stimulation for obstructive sleep apnea: 5-year outcomes. Otolaryngol Head Neck Surg. 2018;159(1):194-202.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





