US Pharm. 2018;43(8)26-33.
ABSTRACT: Alcohol misuse is the third leading preventable cause of death in the United States. Naltrexone is recommended by the American Psychiatric Association, Substance Abuse and Mental Health Services Administration, and the Veterans Affairs/Department of Defense’s most recent published guidelines for the treatment of alcohol use disorder (AUD). Ensuring appropriate use of naltrexone in AUD is an opportunity for pharmacists to be part of an interdisciplinary team in the primary care setting. Keeping up-to-date with the newest guidelines, primary literature, and dosing information is essential for the pharmacist in order to develop and monitor a naltrexone treatment plan in AUD patients.
Alcohol use disorder (AUD) is a chronic relapsing brain disease characterized by the compulsive and uncontrollable use of alcohol. AUD is diagnosed using the Diagnostic and Statistical Manual of Mental Disorders, Fifth edition (DSM-5).1 DSM-5 lists a series of 11 criteria describing alcohol use. The presence of two or more of these listed criteria results in a diagnosis of AUD, either mild, moderate, or severe (see Table 1).1
According to the 2015 National Survey on Drug Use and Health, of the 15.1 million American adults aged 18 years and older, roughly 6.2%, or 936,000, of these individuals met criteria for AUD.2,3 Alcohol misuse is the third leading preventable cause of death in the United States.2,4 An estimated 88,000 people die annually from alcohol misuse; it is the third leading preventable cause of death in the U.S.2,4 Excessive alcohol consumption, including binge drinking and heavy drinking, continues to present a significant financial burden, costing the U.S. $249 billion in 2010.2,5 The three costliest components are lost productivity, mortality, and healthcare.5
AUD is treated in a variety of settings, including primary care, hospitals, and specialized outpatient treatment centers.6 Primary care, specifically, is an important setting in which to screen for adult AUD, according to the May 2013 United States Preventive Services Task Force recommendations.7 Once identification and assessment of at-risk drinking and AUD are completed, the primary care provider may choose to start a medication treatment according to clinical practice guidelines or to refer the patient for management by an addiction or psychiatric specialist.
AUD treatment in primary care settings has been found to be effective when the following interventions are used alone or in combination: substance abuse counseling, social support groups (i.e., Alcoholics Anonymous), and pharmacotherapy. Primary care providers tend to have high rates of patient engagement, increased patient trust, and access to long-term follow-up.6 The community pharmacist can serve as a valuable resource to primary care providers who may be hesitant to prescribe naltrexone owing to a lack of knowledge of the medication or a poor understanding of the disorder and the treatment options available.8 Clinical pharmacists in primary care clinics can play an important role in providing pharmacotherapeutic information and assist in meeting the goal of improving outcomes in AUD patients.
In order to manage AUD effectively, clinical pharmacists should be knowledgeable about the available pharmacologic treatment options recommended by the current consensus AUD guidelines. There are currently three consensus practice guidelines for the treatment and management of AUD published by the following organizations: the American Psychiatric Association (APA), the Substance Abuse and Mental Health Services Administration (SAMHSA), and the Veterans Affairs/ Department of Defense (VADoD).9-11 This article will highlight important naltrexone prescribing information and review the three consensus guidelines addressing the use of naltrexone.
NALTREXONE
Naltrexone is a pure opioid antagonist that competitively binds to mu-opioid receptors, thereby blocking the effects of opioids.12 Endogenous opioids may be involved in alcohol’s reinforcing effects.13 By blocking the opioid receptors, naltrexone may reduce alcohol cravings in the AUD patient.14 Additionally, naltrexone has been found to reduce drinking days in patients with AUD and has demonstrated benefits for those who struggle with abstinence.9
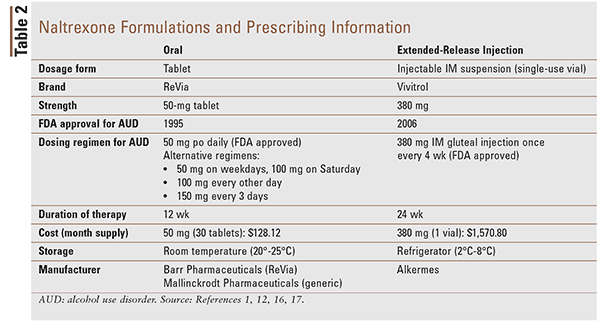
The FDA approved oral naltrexone in 1995 for the treatment of alcohol dependence.15 Extended-release IM naltrexone (Vivitrol) was approved by the FDA and became available in 2006. The clinical effectiveness of IM naltrexone compared with oral naltrexone for AUD in randomized head-to-head clinical trials is unknown.9 A summary of prescribing information regarding both dosage forms of naltrexone is described in Table 2.1,12,16,17
Oral naltrexone 50 mg is a well-tolerated adjuvant for treatment of AUD. The most common adverse event is nausea, reported in 10% to 33% of patients.12 Administration with food or after meals may minimize adverse gastrointestinal effects.12 IM naltrexone’s side-effect profile varies based on the route of administration. Beginning with administration of the extended-release IM formulation, naltrexone injection sites should be monitored for reactions (69%) including injection-site pain, tenderness, induration, swelling, erythema, bruising, or pruritus.17 Clinicians must also monitor for development of depression or suicidal thinking in the patient taking either naltrexone formulation (8%-10%).12 Naltrexone is a Pregnancy Category C risk factor and is excreted into the breast milk.12,16,17
Both the oral and injectable forms of naltrexone carry FDA black box warnings for hepatotoxicity.12,16,17 Hepatotoxicity was found to be associated with doses three to six times the recommended 50- to 100-mg doses.16 Naltrexone is contraindicated in patients with acute hepatitis or liver failure and should be used with caution in patients with active liver disease.12,16,17 Patients should be warned of the risk of hepatic injury and to seek medical attention if they experience associated symptoms.12,16,17
Naltrexone has the potential to exacerbate withdrawal symptoms in patients who are not free of exogenous opioids.12,16,17 Pharmacists should remind prescribers of the importance of patients being completely opioid-free for 7 to 10 days before initiating therapy with naltrexone, in order to decrease the potential for adverse events.9,12,16,17 Administration of a naloxone challenge test or a urine screen for opioids should be performed before naltrexone is prescribed.12,16,17 Patients transitioning from buprenorphine or methadone may be vulnerable to precipitation of withdrawal symptoms for up to 2 weeks.16 Once patients have been treated with naltrexone, they may respond to lower opioid doses than previously used.12,16,17
CLINICAL PRACTICE GUIDELINES
American Psychiatric Association
The most recent of the three clinical-practice guidelines addressing pharmacotherapy for AUD was published in January 2018 by the APA.9 The goal of this guideline is to improve treatment outcomes and quality of care in AUD patients.9 This extensive and detailed guideline was developed by physicians and experts in the field of substance abuse who used the Grading of Recommendations Assessment, Development, and Evaluation process of rating research on the strength of evidence.9 The guideline statements were developed based on a balance of benefits versus harms from clinical-trials data, expert opinion, and patient values and preferences.9
The APA guideline addresses all available pharmacotherapeutic treatments for AUD.9 Naltrexone is recommended as a suitable pharmacologic option to treat moderate-to-severe alcohol abuse and dependence.9 Naltrexone has the best available evidence for the treatment of AUD, according to the guideline, and the APA recommends that naltrexone be offered to moderate-to-severe AUD patients who: 1) have the goal of achieving abstinence or reducing alcohol use, 2) have not responded to nonpharmacologic treatments alone or prefer pharmacotherapy, and 3) have no contraindications to naltrexone.9 The overall strength of evidence for the efficacy of naltrexone was found to be moderate based on the large number of randomized, controlled trials of the drug.9 The APA noted that there were inconsistences between the studies.9
This guideline does not provide recommendations on the preferred route of administration because no head-to-head comparisons of the two naltrexone formulations have been completed.9 The APA guideline does note that long-acting IM naltrexone may improve adherence, but oral therapy may be appropriate if compliance is not a concern.9 Pill counts or a review of the prescription-refill profile may be helpful in assessing compliance. The typical oral naltrexone dosage is 50 mg daily, with a range of 50 mg to 150 mg; the injectable dosage range is 150 mg to 400 mg IM every 4 weeks.9
This guideline also recommends against the use of naltrexone in acute hepatitis, hepatic failure, and those using opioids.9 The APA advises that naltrexone may be prescribed in patients with AUD and co-occurring opioid-use disorder who are abstaining.9
Substance Abuse and Mental Health Services Administration
In 2015, SAMHSA published the 35-page document Medication for the Treatment of Alcohol Use Disorder: A Brief Guide.10 Panel members included physicians and scientists from multiple U.S. government agencies, universities, and consultant groups.10 This clinical guide references primary literature and discusses AUD medications.10 Unlike the APA, SAMHSA does not reveal the methodology used for recommendations provided by the consensus panel.10 The guide reviews screening for risky alcohol use by the clinician and assessing the need for medication-assisted treatment (MAT) with any of the four FDA-approved medications—disulfiram, naltrexone, and acamprosate tablets and naltrexone injection—using patient history, physical examination, and laboratory testing.10 The guide supports goal setting for MAT, developing the individualized treatment plan, and monitoring patient progress.10
The SAMHSA guideline recommends that oral naltrexone be prescribed for AUD patients who are highly motivated and/or supported with observed daily dosing.10 It specifies that naltrexone is most effective in those who are abstinent from alcohol consumption.10 SAMHSA reports that naltrexone is effective for those with a family history of AUD, patients with an intense craving for alcohol, and AUD patients with opioid-use disorder.10 Dosing recommendations include injectable naltrexone 380 mg IM every 30 days, alternating gluteal side for each injection, and oral naltrexone dosing of 50 mg daily in those who are highly motivated and/or supported with observed daily dosing.10 The guide reviews naltrexone prescribing information, as well as special considerations in pain management and co-occurring disorders.10 According to the SAMHSA guideline, the optimal duration of naltrexone treatment is unknown but could be 6 to 12 months.10 Some individuals require only brief treatment during stressful periods known to trigger alcohol cravings.10
Veteran Affairs and Department of Defense
In 2015 the VADoD published a clinical practice guideline for the management of substance-use disorders.11 This 169-page guideline addresses evidence-based pharmacotherapy recommendations for AUD and opioid-use disorder.11 Either oral or extended-release IM naltrexone is recommended as a first-line pharmacotherapy option for the treatment of moderate-to-severe AUD.11 These guidelines report that naltrexone cannot be recommended over other available AUD medications because of insufficient comparative evidence.11 Therefore, the AUD pharmacotherapeutic choice should be based on patient preferences and contraindications. Similar to the APA guideline, the VADoD guideline states that the naltrexone formulations have not been directly compared to evaluate efficacy and clinical outcomes.11 The VADoD recommends that injectable naltrexone be considered only if medication adherence is of significant concern.11 Additionally, the patient must be willing to agree to a treatment that requires monthly injections by a provider.11 The VADoD guideline also recommends at least 3 to 5 days of pretreatment alcohol abstinence for improved response to naltrexone.11
ALTERNATIVE USE OF NALTREXONE
One naltrexone dosing regimen not recommended in any of the AUD pharmacotherapy practice guidelines is the Sinclair Method. There is limited research on this nearly 20-year-old dosing regimen, but it is vital for the clinical pharmacist to be familiar with this non–FDA-approved dosing approach because people may present with questions regarding the use of naltrexone on an as-needed basis based on alternative dosing strategies discussed in layperson chat rooms and websites. The pharmacist may notice inconsistent refills of naltrexone, which is a possible indication that the Sinclair Method is being trialed. Sinclair developed and published this prescribing method based on preclinical research and the concept of pharmacological extinction.18 His patients were instructed to take oral naltrexone as needed prior to drinking, with the goal of reducing alcohol consumption and cravings.18 The Sinclair Method does not require prior detoxification from alcohol before naltrexone initiation.18 There is some overlap between Sinclair’s work regarding the development of his method and naltrexone research studies and the previously discussed AUD practice guidelines.18 In a 2017 article discussing the importance of prevention as a component of public health, the Sinclair Method was cited as an example.19
CONCLUSION
AUD is an ongoing chronic health issue in the U.S. Recent guidelines recommend the use of naltrexone in the treatment and management of AUD. The APA, SAMHSA, and VADoD guidelines have similar and overlapping recommendations for the use of naltrexone in AUD. Naltrexone has proven efficacy in treating AUD and is generally well tolerated. Clinical pharmacists can play a pivotal role as part of an interdisciplinary team in the screening, identification, and management of patients with AUD. Pharmacists can also serve as an important resource on the pharmacotherapeutics of naltrexone for other healthcare professionals and AUD patients. Pharmacists can ensure that naltrexone is prescribed appropriately in this patient population. The use of naltrexone in primary care settings has the potential to decrease morbidity and improve outcomes in patients with AUD.
REFERENCES
1. American Psychiatric Association. Diagnostic And Statistical Manual of Mental Disorders, Fifth ed. Arlington, VA: American Psychiatric Publishing; 2013:490-491.
2. National Institute on Alcohol Abuse and Alcoholism, U.S. Department of Health and Human Services. Alcohol facts and statistics. Updated July 2017. www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/alcohol-facts-and-statistics. Accessed July 16, 2018.
3. Substance Abuse and Mental Health Services Administration. 2015 National Survey on Drug Use and Health (NSDUH). Table 5.6B—Substance use disorder in past year among persons aged 18 or older, by demographic characteristics: percentages, 2014 and 2015.
www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.htm. Accessed January 18, 2018.
4. CDC. Alcohol and public health: alcohol-related disease impact (ARDI). Average for United States 2006–2010 alcohol-attributable deaths due to excessive alcohol use. https://nccd.cdc.gov/DPH_ARDI/default/default.aspx. Accessed January 18, 2018.
5. Sacks JJ, Gonzales KR, Bouchery EE, et al. 2010 national and state costs of excessive alcohol consumption. Am J Prev Med. 2015;49(5):e73-e79.
6. Spithoff S, Kahan M. Primary care management of alcohol use disorder and at-risk drinking: Part 2: counsel, prescribe, connect. Can Fam Physician. 2015;61(6):515-521.
7. United States Preventive Services Task Force. Final recommendation statement: alcohol misuse: screening and behavioral counseling interventions in primary care. Published May 2013. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/alcohol-misuse-screening-and-behavioral-counseling-interventions-in-primary-care. Accessed January 18, 2018.
8. Jonas D, Amick H, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900.
9. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175:86-90.
10. Substance Abuse and Mental Health Services Administration and National Institute on Alcohol Abuse and Alcoholism. Medication for the treatment of alcohol use disorder: a brief guide. HHS Publication No. (SMA) 15-4907. Rockville, MD: Substance Abuse and Mental Health Services Administration. Published 2015.
11. U.S. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guidelines for the management of substance use disorders. Veterans Health Administration, The Management of Substance Use Disorders Work Group. Version 3.0—2015. December 2015. www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPGRevised22216.pdf. Accessed July 14, 2018.
12. Naltrexone. Lexi-Drugs Online. Hudson, Oh: Lexi-Comp, Inc; 2018. https://online.lexi.com. Accessed July 15, 2018.
13. Hemby SE, Johnson BA, Dworkin SI. Neurobiological basis of drug reinforcement. In: Johnson BA, Roache JD, eds. Drug Addiction and Its Treatment: Nexus of Neuroscience and Behavior. Philadelphia, PA: Lippincott-Raven; 1997:137-169.
14. Maisel NC, Blodgett JC, Wilbourne P, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108(2):275-293.
15. Harris AHS, Kivlahan DR, Bowe T, Humphreys KN. Pharmacotherapy of alcohol use disorders in the Veterans Health Administration. Psychiatr Serv. 2010;61:392-398.
16. ReVia (naltrexone) package insert. Pomona, NY: Barr Pharmaceuticals; 2013.
17. Vivitrol (naltrexone for extended-release injectable suspension) package insert. Waltham, MA: Alkermers; 2010.
18. Sinclair J. Evidence about the use of naltrexone and four different ways of using it in the treatment of alcoholism. Alcohol Alcohol. 2001;36(1):2-10.
19. Manjunatha N. Preventive psychiatry from public health perspectives. Indian J Soc Psychiatry. 2017;33:148-152.
To comment on this article, contact rdavidson@uspharmacist.com.