US Pharm. 2017;42(1):HS16-HS20.
ABSTRACT: Neuromuscular blocking agents (NMBAs) play an important role in the management of a large number of hospital patients. The pharmacology of NMBAs is well understood, but the use of these agents can be controversial. NMBAs are common in surgical situations and rapid sequence intubation, but other indications, such as acute respiratory distress syndrome, therapeutic hypothermia, and elevated intracranial pressure, are somewhat divisive. It is essential for pharmacists to be familiar with clinical implications and outcomes associated with the use of NMBAs. In addition, it is important to understand concurrent considerations such as sedation, monitoring, and reversal. Pharmacists in the hospital setting should be familiar with the recently approved novel direct-reversal agent sugammadex (Bridion).
Neuromuscular blocking agents (NMBAs) play an important role in the management of a large number of hospital patients. In addition to their routine use in surgical anesthesia, NMBAs may be valuable in many new and evolving critical care situations. Therefore, it is essential for the hospital pharmacist to become familiar with the clinical implications and outcomes associated with NMBA use and reversal.
Pharmacology
NMBAs exert their pharmacologic effects by modulating signal transmission in skeletal muscle. Action potentials (changes in electrical potential associated with the passage of an impulse along the membrane of a muscle or nerve cell) reaching skeletal muscle activates the release of acetylcholine into the motor endplates. Acetylcholine binds to nicotinic receptors at the endplate, resulting in the release of Na+ (sodium) into muscle fibers, which triggers the muscular action potential. Calcium ions are then released into the sarcoplasmic reticulum, provoking the binding of myosin to actin. Myosin will continue to bind and move along actin sites, shortening the sarcomere, as long as calcium is present in the cell. NMBAs work in two ways to block this process.
Depolarizing NMBAs act as agonists at nicotinic receptors.1 They hold open the ion-gated channels, leading to muscular fasciculation until the ion potential is depleted, and then to paralysis.2 Succinylcholine is the only depolarizing NMBA available. Nondepolarizing NMBAs are competitive antagonists at nicotinic receptors, blocking acetylcholine at the motor endplate.1 This prevents the action potential from spreading, thereby rendering muscle cells insensitive to motor nerve impulses. Muscle paralysis occurs sequentially, beginning with small, fast-twitch muscles in the eyes and larynx and progressing to the limbs, trunk, airway, intercostal muscles, and diaphragm. Recovery from neuromuscular blockage occurs in the reverse order.2
As a result of their mechanistic effects on acetylcholine, NMBAs exhibit many side effects. Acetylcholine plays a role in histamine release, muscarinic activation, vagolytic action, and norepinephrine release. As a result, side effects such as tachycardia and bradycardia, hypertension and hypotension, and bronchodilation and bronchospasm have been seen with their use (TABLE 1).
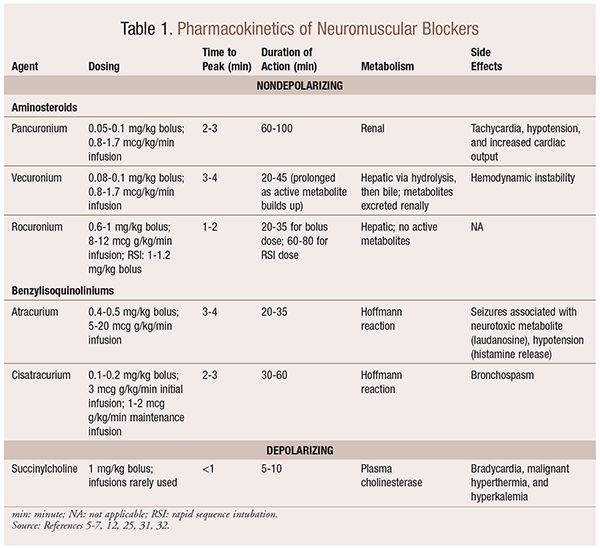
Pharmacokinetics
Depending on the situation, it is useful to have a variety of kinetic profiles available when an NMBA is being selected. Rapid onset and short duration are useful for indications such as rapid sequence intubation (RSI), whereas those with a longer duration are of more value in surgery. See TABLE 1 for a brief overview of kinetic profiles, including dosing and common side effects.
Clinical Usage
Surgical: NMBAs have been a staple of anesthesiology and surgery since the introduction of succinylcholine in 1952.3-7 The choice of agent and dosing varies widely depending on the surgical procedure and also on the use of alternative agents, including general anesthetics, local anesthetics, and IV sedation medications. Of primary concern in the surgical use of NMBAs is to achieve appropriate levels of muscular blockade without inducing cardiovascular side effects or lengthening the total duration of blockade beyond the time frame of the surgical procedure.4 Although a full discussion of agent selection and dosing for surgical indications is beyond the scope of this article, the clinical reversal of these effects will be addressed. Clinical monitoring of a patient who has received an intermediate- to long-acting NMBA during a surgical procedure should include peripheral nerve stimulation (PNS) testing (discussed later).1,4
RSI: RSI is an emergent process used to secure the airway of an unstable patient. RSI protocols involve the administration of a deep-sedation induction agent (e.g., propofol, etomidate, midazolam) with the near-simultaneous administration of an NMBA. The desired outcome is for the patient to develop both amnestic sedation and profound muscle relaxation, thereby improving the likelihood of a successful intubation.8,9 The selection of appropriate agents should be based on agent-specific pharmacokinetics and patient-specific clinical variables.
An ideal agent for RSI is one with both a rapid onset and a rapid offset, which decreases the time from administration to intubation and also reduces the overall duration of paralysis.10 A long delay in drug onset increases the overall risk of hypoxia for the patient. As the onset time lengthens, more time must pass between the last breath via the bag and the first breaths delivered via the endotracheal tube. Increased duration of paralysis heightens the risk of failed intubation.10,11 Instead of the rapid recovery seen with succinylcholine, longer-duration agents such as rocuronium can require respiratory support for more than 90 minutes.12
A large Cochrane review published in 2015 demonstrated that succinylcholine was superior to rocuronium with regard to favorable intubation conditions. These findings likely reflect the favorable kinetic profile of succinylcholine.13 However, several important contraindications to the use of succinylcholine exist in clinical practice. In patients with a history of malignant hyperthermia or in those at high risk for developing hyperkalemia, rocuronium remains a viable RSI agent.
Critical Care
Acute Respiratory Distress Syndrome (ARDS): One of the primary clinical concerns for patients who develop ARDS is to reduce pressure and stress on the lungs, thereby reducing additional inflammation beyond the initial damage or insult. Because of their effects on diaphragmatic tone, NMBAs have been suggested as a method for decreasing ventilator asynchrony and pulmonary pressures. Three primary studies have been published in English that evaluated the use of NMBAs in the early phase of ARDS.14-16 Each study demonstrated increased oxygenation in patients treated early with a 48-hour continuous infusion of cisatracurium. A 2013 meta-analysis of pooled data from these studies showed that early administration of cisatracurium resulted in reduced barotrauma (P = .02) and decreased hospital mortality (P = .005), but had no effect on duration of mechanical ventilation (P = .57).17
Based on these findings, it is reasonable to consider NMBAs for the treatment of acute ARDS in patients presenting to the ICU.4 While the mechanism of action for all nondepolarizing NMBAs should contribute to the proposed mechanism of clinical benefit in ARDS, published randomized, controlled trials used cisatracurium only in the interventional group. Whether other agents would have an effect on these outcomes has not been addressed in the literature.
Therapeutic Hypothermia: NMBAs have been proposed as part of many treatment algorithms for patients undergoing therapeutic hypothermia post cardiac arrest.18 By controlling shivering, NMBAs may decrease total oxygen consumption. Although this has not been studied in critical care, these physiologic changes have been demonstrated in surgical patients undergoing hypothermia during cardiopulmonary bypass.19,20 In a retrospective study of 111 patients who underwent therapeutic hypothermia (18 with NMBA vs. 93 without), there was an improvement in hospital survival (P = .004) that remained significant after multivariate analysis of potential baseline confounders (95% CI, 1.56-33.38).21 A trend toward improvements in lactate clearance and functional outcomes was also noted in patients receiving continuous NMBA.
Another retrospective study addressed the selection of NMBAs in patients undergoing therapeutic hypothermia post cardiac arrest.22 Using a multivariate analysis, the researchers compared 201 patients receiving therapeutic hypothermia (29.9% with cisatracurium vs. 17.9% with vecuronium). Cisatracurium was the only independent positive predictor of survival with good neurologic outcome (P = .014). Owing to the retrospective nature and small sample size of this study, it is difficult to assess the overall power to detect any difference offered by vecuronium similar to what was observed with cisatracurium. It is possible that confounding variables may have a stronger influence than the selected neuroblockade agent on overall outcomes.22
Elevated Intracranial Pressure (ICP): ICP is routinely managed by deep sedation and analgesia, reducing oxygen consumption and cerebral metabolism while controlling pain, motion, and ventilator asynchrony.2 The addition of NMBAs is considered only when deep sedation is insufficient for controlling dangerous increases in ICP, often a result of coughing, suctioning, or shivering.2,12 Early NMBA use has been shown to lead to decreased mortality, but at the cost of increased morbidity. Hsiang and colleagues retrospectively reviewed data on a cohort of 514 patients from the Traumatic Coma Data Bank to evaluate the use of early extended NMBA use versus short-duration NMBA use. ICU stay was an average of 3 days longer, more patients developed pneumonia, and there was a trend toward increased risk of sepsis in extended-NMBA patients. Additionally, although there were more deaths in the non-NMBA group, the NMBA group had a greater incidence of vegetative or severely disabled survivors.23 Use of NMBAs in increased ICP leads to significant difficulties in monitoring neurologic function and seizure activity.24
Reversal Agents
Although uncommon in the ICU, reversal of NMBAs is an important part of the surgical management of patients receiving paralytics. Historically, this has occurred through the use of neostigmine postoperatively.4 Neostigmine is an acetylcholinesterase inhibitor (ACheI) that reduces the breakdown of acetylcholine in the motor endplate, causing an increase in the concentration of acetylcholine. Because nondepolarizing NMBAs are competitive antagonists of the nicotinic receptor, neostigmine increases the competitive pressure of acetylcholine at the site of drug action. Although neostigmine is effective at improving recovery times post NMBA administration, it can be unreliable because of its indirect mechanism of action.4,25 If NMBA concentrations are high enough, the antagonism cannot be overcome regardless of the anticholinesterase dose administered. Failure to fully reverse NMBAs postoperatively has been shown to increase the rates of residual weakness and dysphagia and the risk of aspiration.4,25 Additionally, neostigmine has its own side effects through its action on both the nicotinic and the muscarinic receptors. Whereas the nicotinic receptor is blocked via the NMBA, neostigmine’s effect on the muscarinic receptor results in increased bronchospasms, gastric motility, secretions, and bradycardia.25 To reduce these risks, an antimuscarinic agonist such as atropine or glycopyrrolate must be used with neostigmine to offset the increased muscarinic activation.
In December 2015, the FDA approved sugammadex (Bridion), a novel direct-reversal agent for rocuronium and vecuronium. Classified as a gamma-cyclodextrin, sugammadex creates a drug-drug complex with free NMBA, thereby reducing the available agent concentrations. These pharmacokinetic effects on NMBA concentration are both rapid and complete. However, because of their size, cisatracurium, atracurium, and succinylcholine are unaffected by sugammadex.26 The primary benefit of sugammadex, compared with neostigmine, is the speed at which reversal occurs (2 minutes vs. 17 minutes).27 Unlike ACheIs, sugammadex has no ceiling effect; i.e., with a large enough sugammadex dose, any depth of paralysis can be rapidly reversed.26,28 Lastly, the use of sugammadex has no pharmacodynamic effect on muscarinic receptors, significantly lessening the medication’s side-effect profile and avoiding the need to coadminister antimuscarinic agents. Dosing of sugammadex is based on level of neuromuscular blockade, ranging from 2 mg/kg for moderate blockade to 16 mg/kg for immediate reversal.26,28
Sedation
It is extremely important to recognize that although NMBAs prevent muscular movement, they have no effect on the patient’s level of consciousness or ability to perceive pain or discomfort, resulting in a phenomenon described as unintentional awareness.1 Whether for surgical or for medical indications, patients may be at risk for insufficient sedation or analgesia. A case series demonstrated that up to 18% of patients maintain some degree of awareness while paralyzed.29 These patients report sensations ranging from a dreamlike state to explicit awareness. There are no trials providing specific guidance on the management of unintentional awareness during neuromuscular blockade. Regardless, it is considered standard practice to establish and maintain appropriate levels of analgesia and deep sedation prior to and during neuromuscular blockade.1
Monitoring
The monitoring of patients on NMBAs is imperative, but methods are often complicated by clinical course, coadministration of sedatives and analgesics, and additional therapeutic modalities (e.g., therapeutic hypothermia). PNS is commonly regarded as the monitoring method of choice, but it has limitations. Additional monitoring parameters include spontaneous breathing and trends in vital signs.2
PNS is recommended independently for patients on a continuous infusion of NMBAs and as an adjunct in other clinical situations.1 A train-of-four device attached at the orbicularis oculi, ulnar nerve, or peroneal nerve delivers four successive electrical stimuli. In the absence of neuromuscular blockade, an equal twitch response will be observed for all four stimuli, resulting in a T4/T1 ratio of 1. In a paralyzed patient, the twitch response should be observed with the first stimulus, but this should diminish because of the blockade of motor neurons. The goal is to achieve a T4/T1 ratio of approximately 0.25.1
Complications
Although there are significant benefits to the use of NMBAs in particular situations, there are also short-term and long-term complications. In the acute setting, the use of NMBAs can lead to increased ICU stay, prolonged mechanical ventilation, venous thromboembolism, skin tearing and ulcerations, infection, corneal damage, and anaphylaxis. Long-term administration can lead to immobility or increased recovery time because of impaired neuromuscular transmission and muscular weakness.30 Several recommendations for preventing these effects are outlined in TABLE 2.
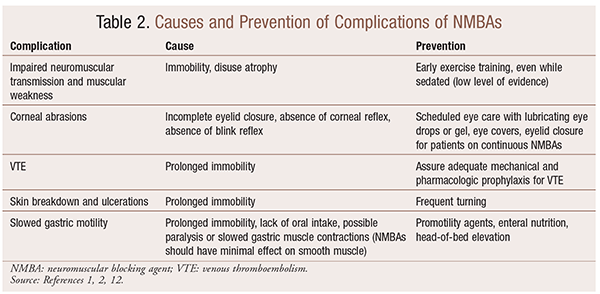
Conclusion
The pharmacist can play a highly important role in the regulation and use of NMBAs across a wide range of clinical-practice sites. By understanding the mechanism of action, therapeutic indications, supporting literature, and clinical side effects of this high-alert class of medications, the pharmacist can have an invaluable effect on patient care and patient safety.
REFERENCES
1. Murray MJ, DeBlock H, Erstad B, et al. Clinical practice guidelines for sustained neuromuscular blockade in the adult critically ill patient. Crit Care Med. 2016;44:2079-2103.
2. Warr J, Thiboutot Z, Rose L, et al. Current therapeutic uses, pharmacology, and clinical considerations of neuromuscular blocking agents for critically ill adults. Ann Pharmacother. 2011;45:1116-1126.
3. Foldes FF, McNall PG, Borrego-Hinojosa JM. Succinylcholine: a new approach to muscular relaxation in anesthesiology. N Engl J Med. 1952;247:596-600.
4. Naguib M, Lien CA. Pharmacology of muscle relaxants and their antagonists. In: Miller’s Anesthesia. 7th ed. Miller RD, Eriksson LI, Fleisher LA, et al, eds. Philadelphia, PA: Churchill Livingstone; 2010.
5. Anectine (succinylcholine) package insert. Princeton, NJ: Sandoz Inc; September 2010.
6. Rocuronium package insert. Irvine, CA: Teva Parenteral Medicines, Inc; November 2008.
7. Nimbex (cisatracurium) package insert. North Chicago, IL: Abbott Laboratories; 2010.
8. Sakles JC, Laurin EG, Rantapaa AA, Panacek EA. Airway management in the emergency department; a one-year study of 610 tracheal intubations. Ann Emerg Med. 1998;31:325-332.
9. Tayal VS, Riggs RW, Marx JA, et al. Rapid-sequence intubation at an emergency medicine residency: success rate and adverse events during a two-year period. Acad Emerg Med. 1999;6:31-37.
10. Li J, Murphy-Lavoie H, Bugas C, et al. Complications of emergency intubation with and without paralysis. Am J Emerg Med. 1999;17:141-143.
11. Naguib M, Samarkandi AH, El-Din ME, et al. The dose of succinylcholine required for excellent endotracheal intubating conditions. Anesth Analg. 2006;102:151-155.
12. Greenberg SB, Vender J. The use of neuromuscular blocking agents in the ICU: where are we now? Crit Care Med. 2013;41:1332-1344.
13. Tran DT, Newton EK, Mount VA, et al. Rocuronium versus succinylcholine for rapid sequence induction intubation. Cochrane Database Syst Rev. 2015;(10):CD002788.
14. Gainnier M, Roch A, Forel JM, et al. Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Crit Care Med. 2004;32:113-119.
15. Forel JM, Roch A, Marin V, et al. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Crit Care Med. 2006;34:2749-2757.
16. Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107-1116.
17. Alhazzani W, Alshahrani M, Jaeschke R, et al. Neuromuscular blocking agents in acute respiratory distress syndrome: a systemic review and meta-analysis of randomized controlled trials. Crit Care. 2013;17:R43.
18. Chamorro C, Borrallo JM, Romera MA, et al. Anesthesia and analgesia protocol during therapeutic hypothermia after cardiac arrest: a systematic review. Anesth Analg. 2010;110:1328-1335.
19. Cruise C, MacKinnon J, Tough J, Houston P. Comparison of meperidine and pancuronium for the treatment of shivering after cardiac surgery. Can J Anaesth. 1992;39:563-568.
20. Sladen RN, Berend JZ, Fassero JS, et al. Comparison of vecuronium and meperidine on the clinical and metabolic effects of shivering after hypothermic cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 1995;9:147-153.
21. Salciccioli JD, Cocchi MN, Rittenberger JC, et al. Continuous neuromuscular blockade is associated with decreased mortality in post-cardiac arrest patients. Resuscitation. 2013;84:1728-1733.
22. Baker WL, Geronila G, Kallur R, et al. Effect of neuromuscular blockers on outcomes in patients receiving therapeutic hypothermia following cardiac arrest. Analg Resusc Curr Res. 2013;S1.
23. Hsiang JK, Chesnut RM, Crisp CB, et al. Early, routine paralysis for intracranial pressure control in severe head injury: is it necessary? Crit Care Med. 1994;22:1471-1476.
24. Rangel-Castilla L, Gopinath S, Robertson CS. Management of intracranial hypertension. Neurol Clin. 2008;26:521-541.
25. Caldwell JE. Clinical limitations of acetylcholinesterase antagonists. J Crit Care. 2009;24:21-28.
26. Bridion (sugammadex) package insert. Whitehouse Station, NJ: Merck & Co, Inc; July 2015.
27. Sacan O, White PF, Tufanogullari B, Klein K. Sugammadex reversal of rocuronium-induced neuromuscular blockade: a comparison with neostigmine-glycopyrrolate and edrophonium-atropine. Anesth Analg. 2007;10:569-574.
28. Meistelman C, Donati F. Do we really need sugammadex as an antagonist of muscle relaxants in anesthesia? Curr Opin Anaesthesiol. 2016;29:462-467.
29. Arnot-Smith J, Smith AF. Patient safety incidents involving neuromuscular blockade: analysis of the UK National Reporting and Learning System data from 2006 to 2008. Anaesthesia. 2010;65:1106-1113.
30. Price DR, Mikkelsen ME, Umscheid CA, Armstrong EJ. Neuromuscular blocking agents and neuromuscular dysfunction acquired in critical illness: a systematic review and meta-analysis. Crit Care Med. 2016;44:2070-2078.
31. Prielipp RC, Coursin DB, Scuderi PE, et al. Comparison of the infusion requirements and recovery profiles of vecuronium and cisatracurium 51W89 in intensive care unit patients. Anesth Analg. 1995;81:3-12.
32. Sparr HJ, Wierda JM, Proost JH, et al. Pharmacodynamics and pharmacokinetics of rocuronium in intensive care patients. Br J Anaesth. 1997;78:267-273.
To comment on this article, contact rdavidson@uspharmacist.com.






