US Pharm. 2017;42(1):25-27.
Attention-deficit hyperactivity disorder (ADHD) is a condition that affects the brain. A persistent pattern of inattention and/or hyperactivity with impulsivity is remarkable in most patients.1 Such patterns are key factors when establishing a diagnosis. Patients with ADHD are typically delineated into three general categories: predominantly inattentive, predominantly hyperactive-impulsive, and combined.1,2
Predominantly Inattentive Presentation: Patients with predominantly inattentive ADHD typically forget details of daily routines and are easily distracted. It is also difficult for such patients to organize or finish a task, pay attention to details, or follow instructions.2
Predominantly Hyperactive-Impulsive Presentation: Patients with a hyperactive-impulsive presentation often have difficulty waiting for opportunities to participate in tasks or listening to directions.1 Accidents while walking or traveling are more prevalent in these patients. Additionally, individuals tend to fidget and converse abundantly; it appears that it is difficult for them to sit still for an extended period of time.2
Combined Presentation: In those with combined symptoms, inattention and hyperactivity are equally present. In fact, a majority of children with ADHD present with combined symptoms.1
Epidemiology
Though more prevalent in males, ADHD is a common mental disorder that affects 6% to 10% of children.3 It was once considered to be a disease that specifically affected children, as symptoms appeared to decline when patients approached adulthood. However, ADHD is now acknowledged as a condition that affects 50% to 66% of adults.4 The annual societal cost in 2005 was estimated to be between $36 and $52 billion, with an estimated expense of $12,005 to $17,458 per person.5
Diagnosis
Diagnosis of ADHD requires a comprehensive evaluation by a licensed and experienced clinician. Medical classification systems commonly employed include the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) and the International Classification of Mental and Behavioural Disorders 10th revision (ICD-10). Selected DSM-5 diagnostic criteria for ADHD can be found in Table 1.6 Criterion E of the DSM guidelines helps to distinguish ADHD from other mental disorders. In it, the DSM notes that ADHD symptoms should not be better explained by other mental disorders, such as mood, anxiety, dissociative, or personality disorders, and the symptoms should not occur solely during the course of schizophrenia or other psychotic disorders.7

Device
The Neuropsychiatric Electroencephalograph-Based ADHD Assessment Aid (NEBA) system is the first prescription device approved by the FDA to assist in diagnosing ADHD.8,9 The NEBA system uses an electroencephalograph (EEG) to provide an interpretation of the patient’s neuropsychiatric condition.8 Targeting individuals between the ages of 6 and 17, the NEBA system interprets the theta/beta ratio of the EEG, as this ratio has been shown to be higher in children and adolescents with ADHD.8,9 It should be noted that the NEBA system is not used as a stand-alone diagnostic tool and is recommended to be combined with a clinician’s clinical evaluation. The exam usually requires 15 to 20 minutes to complete. A picture of the NEBA System may be found in Figure 1.
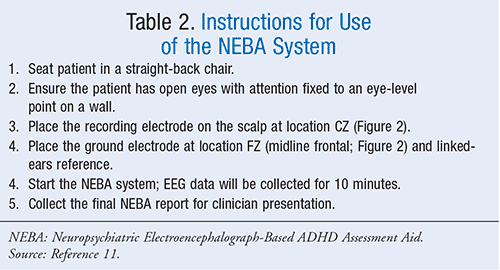
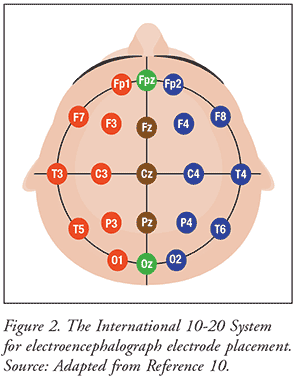
Electrode Application: The International 10-20 system, which is a widely used method for the description and application of EEG scalp electrodes, is used as a basis for electrode placement.8,10 The International 10-20 System for electrode placement and instructions for use (NEBA system) can be seen in Figure 2 and Table 2, respectively.10,11
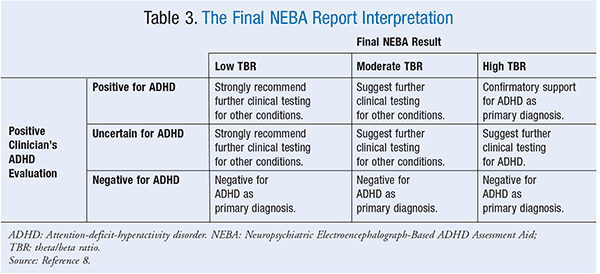
Reporting: Once completed, a NEBA report is forwarded to the clinician’s office, either confirming the diagnosis or suggesting further clinical testing.8 Possible interpretations for the NEBA report may be found in Table 3.11 It should be noted that the NEBA system cannot be used in an individual for whom an EEG recording is invalid. Invalid EEG recordings may be commonly seen in patients with a history of EEG abnormalities or seizure disorders. Other examples of irregularities may include patients taking anticonvulsant medications or who have a metal plate or device in the head.
Efficacy
In a study of 275 children and adolescents presenting with attention and behavioral concerns, 209 subjects met ADHD criteria based on clinician diagnosis. Of the 209 patients, 93 were separately found by the multidisciplinary team (psychiatrist, psychologist, and neurodevelopmental pediatrician) to be less likely to meet criterion E.11 Further, when the NEBA device was used, a relatively lower theta/beta ratio was identified in 85 of the 93 misdiagnosed adolescents, signifying possible over-diagnosis in some patients.
The method used in this study integrated the results of the clinician’s evaluation and the NEBA report. This study demonstrated that the integration method could improve diagnostic accuracy from 61% to 88%.1 The study concluded that 97% of all uncertain cases were found to be in agreement with the results from the multidisciplinary team, validating the efficacy of the NEBA system. Moreover, patients with relatively lower theta/beta ratios were more likely to have other conditions besides ADHD (P <0.05).11
Conclusion
The NEBA system is a complementary tool used to effectively help healthcare practitioners with the clinical diagnosis of ADHD. Additionally, a clinical study indicates that accurate diagnosis is greater when using the device with assessments from a multidisciplinary team. For more information, please contact NEBA Health at (888) 539-4267 or www.nebahealth.com.
REFERENCES
1. National Institutes of Health. National Institute of Mental Health. Attention deficit hyperactivity disorder. Revised March, 2016. www.nimh.nih.gov/health/topics/attention-deficit-hyperactivity-disorder-adhd/index.shtml. Accessed August 11, 2016.
2. CDC. Attention-deficit/hyperactivity disorder (ADHD). Facts about ADHD. Updated November 16, 2016. www.cdc.gov/ncbddd/adhd/facts.html. Accessed September 8, 2016.
3. Seattle Children’s Hospital. Treatments for attention deficit hyperactivity disorder (ADHD) and related problems through personalized medicine and improved diagnosis. www.seattlechildrens.org/research/science-industry-partnerships/partnership-opportunities/adhd. Accessed August 29, 2016.
4. ADHD Institute (Shire PLC). Epidemiology. Site updated December, 2016. www.adhd-institute.com/burden-of-adhd/epidemiology. Accessed December 8, 2016.
5. CDC. Attention-deficit/hyperactivity disorder (ADHD). Data & statistics. Updated October 5, 2016. www.cdc.gov/ncbddd/adhd/data.html. Accessed September 8, 2016.
6. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 2013. Washington, DC: American Psychiatric Association. Cited in: DSM-5 Diagnostic criteria for ADHD. Stepping Stones Psychological Services. https://princetonnassaupediatrics.com/files/dsm-criteria-for-adhd-handout.pdf. Accessed August 30, 2016.
7. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders IV-Text Revision (DSM-IV-TR). 2000. Washington, D.C.: American Psychiatric Association. Cited in: Diagnostic criteria for Attention-Deficit/Hyperactivity Disorder. DSM-IV-TR. http://behavenet.com/node/21488. Accessed August 11, 2016.
8. FDA. De novo classification request for neuropsychiatric EEG-based assessment aid for ADHD (NEBA) system. www.accessdata.fda.gov/cdrh_docs/reviews/K112711.pdf. Accessed August 30, 2016.
9. FDA. FDA permits marketing of first brain wave test to help assess children and teens for ADHD. July 15, 2013. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm360811.htm. Accessed August 30, 2016.
10. 10-20 System Positioning Manual. 2012. Wanchai, Hong Kong: Trans Cranial Technologies. www.trans-cranial.com/local/manuals/10_20_pos_man_v1_0_pdf.pdf. Accessed August 30, 2016.
11. Snyder SM, Rugino TA, Hornig M, Stein MA. Integration of an EEG biomarker with a clinician’s ADHD evaluation. Brain Behav. 2015;5(4)e03330. www.ncbi.nlm.nih.gov/pmc/articles/PMC4356845/. Accessed August 30, 2016.
To comment on this article, contact rdavidson@uspharmacist.com.