ABSTRACT: Interstitial cystitis/bladder pain syndrome (IC/BPS) is a chronic condition of the bladder, which causes pain or discomfort in the absence of infection or other identifiable causes. The exact etiology of IC/BPS is unknown, leading to controversy regarding treatment. The American Urological Association guideline recommends a stepwise approach in the selection of treatment options, based on patient characteristics and the severity of symptoms. Due to the difficulty in fully understanding this condition, the goal of therapy is to provide symptom relief and improve quality of life.
Interstitial cystitis (IC) and bladder pain syndrome (BPS) or painful bladder syndrome are terms that are used together to describe a chronic condition involving bladder pain or discomfort, which can have a significant impact on quality of life. Chronic bladder pain has historically been referred to as interstitial cystitis; however, since there is no clear evidence that bladder inflammation (cystitis) is involved in the pathophysiology or that the condition is associated with abnormalities of the interstices of the bladder, it has been thought to be misnamed.1 The term BPS is not used alone because of the previous efforts to identify IC as a debilitating medical condition, so for continuity reasons it is called IC/BPS.
BACKGROUND
There is controversy surrounding the management of IC/BPS, with no clear consensus for its optimal treatment.1 The symptoms of IC/BPS vary among patients, with the definitions of the condition and how to measure outcomes varying as well. There is also a lack of randomized, controlled trials.2 The American Urological Association (AUA) guideline defines IC/BPS as “an unpleasant sensation (pain, pressure, discomfort) perceived to be related to the urinary bladder, associated with lower urinary tract symptoms of more than six weeks’ duration, in the absence of infection or other identifiable causes.”3
The estimated prevalence of IC/BPS may vary depending on the method used. IC/BPS is more common in women, and a recent estimate shows the female-to-male ratio to be 10:1.1 It is also more common in Caucasians than in other races.1 Among adult women in the United States, the estimated preva-lence is 2.7% to 6.53%.3 IC/BPS is often seen in patients with other pain conditions such as allergies, fibromyalgia, or irritable bowel syndrome.4 According to the National Institutes of Health’s (NIH) 2012 publication, Urologic Diseases in America, annual expenditures exclusive of medication costs for Medicare beneficiaries aged ≥65 years was $249,160,233 (specific and nonspecific IC combined).5
The recommended treatment approach is to use diagnostic criteria to identify the condition and use a stepwise method to aid in choosing the best therapeutic options based on the individual patient characteristics and severity of their symptoms. The AUA has published guidelines on diagnosis and management of IC/BPS; however, they do emphasize that it is a guideline and is not meant to be interpreted rigidly.3
PATHOGENESIS
The etiology and pathogenesis of IC/BPS is not well understood. There may be several factors leading to the manifestation of this disease, and several varying mechanisms have been proposed. Urothelial abnormalities have been found in IC/BPS cases, including an autoimmune or immunologic response causing altered bladder epithelial expression of specific antigens, alterations in the glycosaminoglycan (GAG) layer, and altered cytokeratin profile.1,6 The GAG layer helps protect the bladder from surface irritants, so if it is altered, irritants may leak from the urine into the bladder tissue, resulting in pain and inflammation. In some cases, the bladder wall is scarred or shows petechial hemorrhages known as glomerulations. In approximately 10% of IC/BPS cases, Hunner lesions or patches of broken skin on the bladder wall are present.7
Some information suggests that foods and beverages such as alcohol, caffeine, citrus fruits, tomatoes, and spicy foods may worsen symptoms; however, this is variable from patient to patient.8
SYMPTOMS
The most common symptom is an increase in discomfort with bladder filling and relief with voiding.1 Other symptoms that may be present are urinary urgency, daytime frequency, pain, nocturia, painful voiding, suprapubic pain, perineal pain, sensation of bladder spasms, pubic pressure, dyspareunia, gross hematuria, and depression. Many patients experience a worsening of quality of life and a disruption of home and work activities.1,3
DIAGNOSIS
Diagnosis consists of collecting the patient’s history of symptoms and associated conditions, physical examination, and urine testing. Cystoscopy may be used to exclude other etiologies but is not required to make the diagnosis of IC/BPS. It may be used to identify or rule out other conditions and should be performed if patients are experiencing hematuria. Cystoscopy can also identify structural lesions or an intravesical foreign body, helping to identify a small subset of patients that would benefit from cystoscopic treatment.3,7
Validated symptom scales have been developed to assess IC/BPS severity and clinical progress, but they do not help distinguish IC/BPS from other conditions. There have been several scales used, including the IC Symptom and Problem Index, the Genitourinary Pain Index, and the Pelvic Pain and Urgency/Frequency (PUF) questionnaire.1,3,9
Infection and hematuria should be excluded, so urinalysis with microscopy should be performed on all patients with suspected IC/BPS. A postvoid residual urine volume should also be measured.3
TREATMENT OPTIONS
Since the etiology and pathogenesis are not well understood and there is no curative treatment, the goal of management is to provide relief of symptoms to improve quality of life. The Interstitial Cystitis Data Base study showed that there is no treatment consistently effective in providing relief. In this study, there were 581 women with IC/BPS who underwent 183 different types of treatment over the follow-up period and no single therapy was successful in the majority of study participants.10
IC/BPS is treated in a stepwise approach, and treatments are identified by risk of adverse effects and invasiveness of the treatment (TABLE 1). The management approaches are organized in the order of increasing risk, and clinicians should move from one level to the next when the less risky approach has failed or been found to be ineffective.3
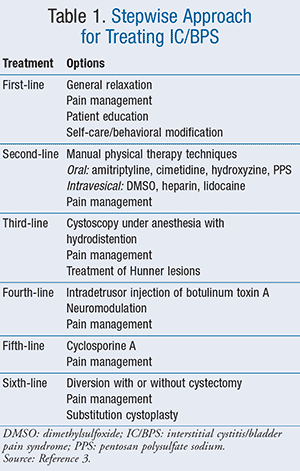
First-Line
Initial treatment should be based on symptom severity, clinician judgment, and patient preferences. Patients should be counseled on reasonable expectations for the treatment outcomes. First-line treatment options consist of patient education, self-care/behavioral modification, general relaxation and stress management, and pain management.3
Patient education on normal bladder function, what is and is not known about IC/BPS, risks and benefits of the available treatment options, the fact that no single agent has been found effective for the majority of patients, and that acceptable symptom control may require trials of multiple options (including combination therapy) before symptom control is achieved is of utmost importance.3
Behavioral modification and self-care practices are also important educational points. These may include altering the volume or concentration of urine by increasing hydration or fluid restriction; applying local heat or cold over the perineum or bladder; avoiding foods known to be irritants; trying an elimination diet to determine which foods may be contributing; use of OTC products (e.g., Pyridium, nutraceuticals, calcium glycerophosphate); mind-body strategies to manage flare-ups (e.g., meditation, imagery); pelvic floor muscle relaxation; and bladder training.3
These strategies have been shown to be effective according to the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) trial that focused on treatment-naïve IC/BPS patients.11 Patients in this trial completed a standardized education and behavioral modification program (EBMP). Forty-five percent of patients (n = 136) assigned to the EBMP with placebo group were markedly or moderately improved on the Global Response Assessment.11
Another first-line treatment option is general relaxation and stress management. Yoga, acupuncture, and hypnosis have been used with variable results.3
Second-Line
Second-line treatment options consist of appropriate manual physical therapy techniques; oral agents such as amitriptyline, cimetidine, hydroxyzine, or pentosan polysulfate sodium (PPS) (TABLE 2); intravesical therapies, including dimethylsulfoxide (DMSO), heparin, or lidocaine; and pain management.3
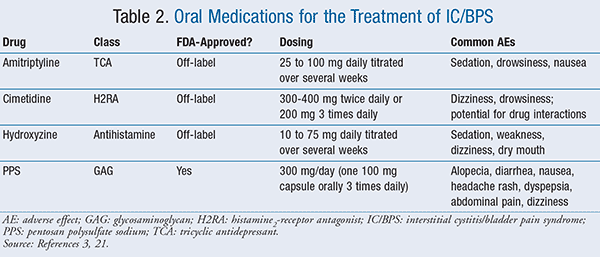
Amitriptyline: One randomized, controlled trial reported oral amitriptyline, a tricyclic antidepressant (TCA), to be superior to placebo when titrated from 25 mg daily to 100 mg daily over several weeks as tolerated.12 Amitriptyline showed a clinically significant improvement in pain and urgency intensity when compared with placebo (P < .001). The most common adverse effects were anticholinergic in nature (i.e., sedation, drowsiness, nausea) and occurred in 92% of patients in the amitriptyline group and 21% in the placebo group.12 If a TCA is used for treatment of IC/BPS, it is important to start with a low dose and titrate slowly, and patients should be educated that it may take several weeks to see a benefit.3
Cimetidine: A histamine2-receptor antagonist (H2RA), cimetidine 400 mg twice daily, was found to be statistically significant to placebo in improving total symptoms of pain and nocturia after 3 months in a randomized, controlled trial.13 There were two other observational studies (300 mg twice daily or 200 mg three times daily) that resulted in 44% to 57% of patients reporting clinically significant improvement in symptoms. No significant adverse effects were reported.14,15 There is a lack of long-term follow-up data, and it is well known that cimetidine has the potential to interact with many other medications.
Hydroxyzine: There is mixed data with the use of hydroxyzine for treatment of IC/BPS. There was a randomized controlled trial that resulted in 23% of patients in the treatment group experiencing clinically significant improvement in pain and urgency compared with 13% in the placebo group over a 6-month period.16 This difference was not found to be statistically significant. Patients were started on 10 mg daily and titrated to 50 mg daily over several weeks.16 There was some improvement in an observational study, in which patients were started on hydroxyzine 25 mg daily and titrated to 75 mg daily over several weeks.17 This study reported 92% of patients experiencing clinically significant improvement in urinary symptoms. Of note, the patients in this study had systemic allergies, which may have resulted in the response to hydroxyzine. Adverse events were common in both studies, and overall they were not serious (e.g., sedation, weakness). It is recommended to give hydroxyzine at bedtime in patients who may have insomnia due to voiding frequently at night. The hypnotic effect of hydroxyzine may be helpful in these patients.17
PPS: This agent, available under the brand-name Elmiron, is the most-studied drug for treatment of IC/BPS, and it is the only oral medication approved by the FDA for treatment of bladder pain or discomfort associated with IC/BPS.18 It has anticoagulant and fibrinolytic properties, and the proposed mechanism of action is acting as a buffer to control cell permeability because it adheres to the mucosal membrane of the bladder wall. This may prevent irritating solutes in the urine from reaching the cells in the bladder wall.18 Per the AUA guideline, PPS receives the highest grade of evidence due to the greatest number of studies, which included more than 500 patients.3
It is important to keep in mind that the trials were fairly high quality but showed mixed results.3 For example, in one randomized, placebo-controlled study of 155 patients, 38% of patients who received PPS (100 mg three times a day for 3 months) and 18% of those who received placebo showed >50% improvement in bladder pain (P = .005).19 However, another randomized, controlled trial reported no significant differences in total symptom scores between PPS (200 mg twice daily for 4 months) and placebo. Both groups had similar rates of clinically significant improvement in symptoms, 56% and 49%, respectively.20
The recommended dosage of PPS is 100 mg three times daily, and it may take 3 to 6 months for relief of symptoms.19 The adverse-effect profile for PPS is favorable (see TABLE 2), making it a good treatment option for patients who have sedation with amitriptyline or antihistamines.19 However, there is currently no generic version of PPS, so it is more costly compared with other oral agents that also have generics available such as TCAs.
Intravesical Treatments: Intravesical drug instillations are second-line options per the AUA guidelines and consist of DMSO, heparin, or lidocaine. Intravesical administration of drugs directly into the bladder allows a high concentration of medication to reach the target area with few systemic side effects. Disadvantages of this method of delivery include cost, risk of infection, and pain from intermittent catheterization.1
DMSO is the only drug approved by the FDA for intravesical drug instillation.3 It is thought to provide relief by having anti-inflammatory, analgesic, smooth muscle relaxation, and mast-cell inhibition effects. It is often given as a cocktail that may include heparin, sodium bicarbonate, a local steroid, and/or a lidocaine preparation. A catheter is placed in the bladder, and DMSO is then passed through the catheter and held for 10 to 15 minutes before normal voiding takes place.3 An example of a cocktail regimen is: 50% DMSO (50 mL), 100 mg hydrocortisone (5 mL), 10,000 units of heparin sulfate (10 mL), and 0.5% bupivacaine (10 mL).21 Treatments are usually given every 1 to 2 weeks for 6 to 8 weeks and repeated as needed.3,7
Pain Management: Options for pain management should be continually assessed because pain plays an important role in quality of life. If pain is not controlled, a multidisciplinary approach should be taken. If patients experience treatment flares, a pain regimen with breakthrough pain management strategies should be considered. The goal is to design a pain regimen that provides significant relief without side effects. Urinary analgesics, nonsteroidal anti-inflammatory drugs (NSAIDs), aspirin, narcotics, and a variety of non-narcotic agents for chronic pain can be used.3
Urinary analgesics consist of phenazopyridine and methenamine. These should be used short term, as long-term use may cause renal or liver dysfunction. Intravesical lidocaine with heparin and/or sodium bicarbonate may be used for those who have acute episodes of severe bladder pain. Because this requires bladder catheterization, it should only be utilized in patients who have not found sufficient relief from other agents.3
Third- to Sixth-Line
If first- and second-line treatments have not helped or patients’ symptoms are worsening, then third-line treatments may be considered. Third-line treatments include cystoscopy under anesthesia with short duration, low-pressure hydrodistention, pain management, and treatment of Hunner lesions, if found.3
Fourth-line treatment options include botulinum toxin A (Botox) injections into the bladder muscle, neuromodulation, and pain management. Botox can cause significant adverse effects, including urinary retention and painful urination. Neurostimulation is not effective for pain but may be useful for urinary frequency/urgency. Fifth-line treatments consist of cyclosporine A with pain management, and sixth-line options are diversion with or without cystectomy, pain management, and substitution cystoplasty.3
SUMMARY
Management of IC/BPS can be challenging given the lack of data and variability in symptoms experienced. The AUA guideline provides a starting point for management, but treatment should be individualized to each patient. Education is key for both providers and patients so there are reasonable expectations. Treatment should consist of both nonpharmacologic and pharmacologic options in a stepwise approach.
REFERENCES
1. McDermott P. Painful bladder syndrome/interstitial cystitis (history, epidemiology, symptoms, diagnosis and treatments). Int J Urol Nurs. 2009;3(1):16-23.
2. Propert KJ, Payne C, Kusek JW, Nyberg LM. Pitfalls in the design of clinical trials for interstitial cystitis. Urology. 2002;60(5):742-748.
3. Hanno PM, Burks DA, Clemens Q, et al. American Urological Association (AUA) guideline. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome. Amended 2014. www.auanet.org/education/guidelines/ic-bladder-pain-syndrome.cfm. Accessed April 25, 2016.
4. Clemens JQ, Meenan RT, O’Keeffe Rosetti MC, et al. Case-control study of medical comorbidities in women with interstitial cystitis. J Urol. 2008;179(6):2222-2225.
5. Chapter 1. Interstitial cystitis and prostatitis. In: Litwin MS, Saigal CS, eds. Urologic Diseases in America. Washington, DC: National Institute of Diabetes and Digestive and Kidney Diseases; 2012. NIH Publication No. 12-7865. https://www.niddk.nih.gov/about-niddk/strategic-plans-reports/Documents/urologic/Trackstar-Urologic%20Diseases%20Chap%2001.pdf. Accessed August 16, 2016.
6. Graham E, Chai TC. Dysfunction of bladder urothelium and bladder urothelial cells in interstitial cystitis. Curr Urol Rep. 2006;7(6):440-446.
7. Interstitial cystitis/painful bladder syndrome. NIDDK. www.niddk.nih.gov/health-information/health-topics/urologic-disease/interstitial-cystitis-painful-bladder-syndrome/Pages/facts.aspx. Accessed May 2, 2016.
8. Friedlander JI, Shorter B, Moldwin RM. Diet and its role in interstitial cystitis/bladder pain syndrome (IC/BPS) and comorbid conditions. BJU Int. 2012;109(11):1584-1591.
9. Clemens JQ, Calhoun, EA, Litwin MS, et al. Validation of a modified National Institutes of Health chronic prostatitis symptom index to assess genitourinary pain in both men and women. Urology. 2009;74(5):983-987.
10. Rovner E, Propert KJ, Brensinger C, et al. Treatments used in women with interstitial cystitis: the Interstitial Cystitis Data Base (ICBD) study experience. The Interstitial Cystitis Data Base Study Group. Urology. 2000;56(6):940-945.
11. Foster HE Jr, Hanno PM, Nickel JC, et al; Interstitial Cystitis Collaborative Research Network. Effect of amitriptyline on symptoms in treatment naïve patients with interstitial cystitis/painful bladder syndrome. J Urol. 2010;183(5):1853-1858.
12. Van Ophoven A, Pokupic S, Heinecke A, Hertle L. A prospective, randomized, placebo controlled, double-blind study of amitriptyline for the treatment of interstitial cystitis. J Urol. 2004;172(2):533-536.
13. Thilagarajah R, Witherow RO, Walker MM. Oral cimetidine gives effective symptom relief in painful bladder disease: a prospective, randomized, double-blind placebo-controlled trial. BJU Int. 2001;87:207-212.
14. Dasgupta P, Sharma SD, Womack C, et al. Cimetidine in painful bladder syndrome: a histopathological study. BJU Int. 2001;88:183-186.
15. Seshadri P, Emerson L, Morales A. Cimetidine in the treatment of interstitial cystitis. Urology. 1994;44(4):614-616.
16. Sant GR, Propert KJ, Hanno PM, et al. A pilot clinical trial of oral pentosan polysulfate and oral hydroxyzine in patients with interstitial cystitis. J Urol. 2003;170(3):810-815.
17. Theoharides TC. Hydroxyzine in the treatment of interstitial cystitis. Urol Clin North Am. 1994;21(1):113-119.
18. Pentosan polysulfate sodium. In-depth answers. Micromedex. www.micromedex.com. Accessed April 28, 2016.
19. Elmiron (pentosan polysulfate sodium) package insert. Titusville, NJ: Janssen Pharmaceuticals, Inc; August 2012.
20. Holm-Bentzen M, Jacobsen F, Nerstrom B, et al. A prospective double-blind clinically controlled multicenter trial of sodium pentosan polysulfate in the treatment of interstitial cystitis and related painful bladder disease. J Urology. 1987;138(3):503-507.
21. Stav K, Beberashvili I, Lindner A, Leibovici D. Predictors of response to intravesical dimethyl-sulfoxide cocktail in patients with interstitial cystitis. Urology. 2012;80(1):61-65.
To comment on this article, contact rdavidson@uspharmacist.com.






