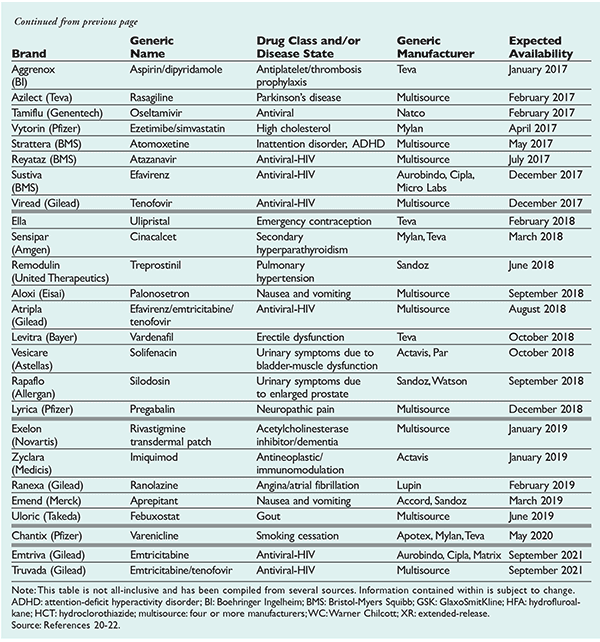
US Pharm. 2016;41(6)(Generics suppl)12-19.
ABSTRACT: Healthcare systems in the United States leverage generic medications as a means to control the pharmaceutical budget with tools that include formulary guidelines, restrictions, and therapeutic interchange programs. Managed care organizations leverage generic medications to improve utilization by carefully selecting tiered formulary pricing. Despite substantial increases in the cost of generic medications over the last 10 years, new multisource generics have the potential to save the U.S. healthcare industry billions of dollars and have a significant therapeutic impact.
Global healthcare costs currently exceed US $1 trillion annually, with generic drugs making up $435 billion of this global market.1 Generic medications offer patients and insurers alike significant monetary savings that in turn contribute to more affordable healthcare markets.2 The acquisition cost of generic drugs for healthcare organizations traditionally ranges from 20% to 80% lower than the innovators’ branded drugs.3,4 Yet in the United States there has been a substantial increase in the cost of generic medications over the last 2 years.5-7 These generic drug prices depend on many market factors that affect supply and demand, including drug shortages and competition.6
In 1984, generic drugs were defined by revisions to the Federal Food, Drug, and Cosmetic Act of 1978 with the Drug Price Competition and Patent Term Restoration Act (known as the Hatch-Waxman Act).8 This simplified the process for ensuring efficacy and safety of new generics by resting an Abbreviated New Drug Application in the original New Drug Application for an innovator’s branded drug. It also provided a naming convention, allowing generic medications to share the “generic” name of the innovator’s drug.
Biosimilars, as defined by the Affordable Care Act (ACA) of 2010 and the Biologics Price Competition and Innovation Act (BPCIA) of 2009, are FDA-approved to market in the U.S. via a different abbreviated pathway and nomenclature.9-11 Biosimilars may or may not afford similar cost savings owing to the extensive development program they require compared to generic medicines, as well as a different naming convention.12,13 Biosimilars are already widely available in Europe. Biosimilars in the U.S. pipeline include antitumor necrosis factor products, short- and long-acting insulins, antineoplastics, colony-stimulating factors, interferons, and erythropoietins.12 Colony-stimulating factors and antitumor necrosis factor products are expected in 2016. Filgrastim (Zarxio) is currently available. Insulin glargine (Basaglar) and infliximab (Inflectra) are FDA-approved but were not yet available at press time. Pegfilgrastim, adalimumab, rituximab, bevacizumab, and trastuzumab are currently in development. This article, however, will focus on traditional generic drugs that are in the pipeline.
By definition, market exclusivity is the time between FDA approval to market a brand drug and the market availability of a generic formulation of that drug.14 Market exclusivity incorporates the life of the patent, the minimum exclusivity period stipulated by the Hatch-Waxman Act, the remaining time on the original patent covering the active principle, and other factors that affect generic entry.15,16
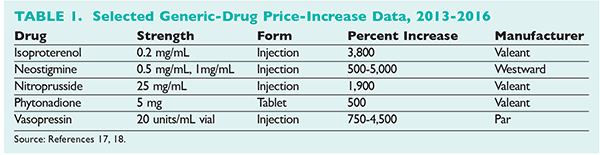
Mergers and acquisitions among generic manufacturers, supply disruption, and increased regulation all contribute to the rising prices of generics (Table 1).17,18 Turing, Valeant, Retrophin, and Rodelis Therapeutics are all being investigated by a Congressional committee for price gouging after purchasing the rights to manufacture and distribute off-patent generic medications.6 Options to promote competitive generic markets in the U.S. have been proposed, as have alternative pricing models for generic drugs.5,7,19
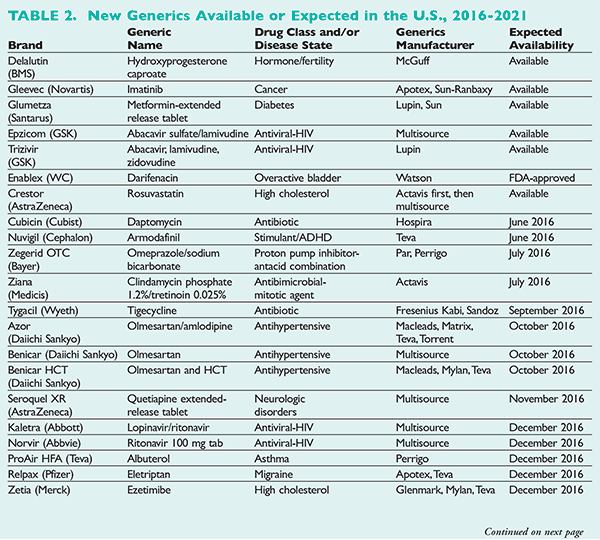
New generics available or expected in the U.S. are listed by date in Table 2.20-22 The price of generics that are available immediately from multisource (four or more) generic manufacturers should be drastically reduced as soon as they come to market. Those generics with 6-month patent exclusivity may be as expensive as their brand innovators’ drugs. Brand-name drugs have an average market exclusivity of 12.5 years.14,23 When sorted by drug class, analgesics exhibit the shortest median effective market exclusivity, while dermatologics, antivirals, and orphan drugs exhibit the longest median market exclusivity.14 Fixed-dose combinations that contain two or more active ingredients extend the patent and exclusivity of the drugs included within.24
A discussion of the major functional drug classes and the new generics that are expected to be available in these classes over the next 48 months follows, with a focus on the financial impact and market share of the highlighted medications, as well as their place in therapy.
Infectious Disease Medications
Daptomycin (Cubicin): Approximately 10% of healthcare bills are attributable to direct costs of antimicrobials, with indirect costs of inappropriate antimicrobial use estimated at $13 million per year per institution.25 Since daptomycin is a broad-spectrum antibiotic, most hospital formularies reserve it for complicated methicillin-resistant Staphlococcus aureus (MRSA) infections. It is a once-a-day infusion or slow IV push and does not require pharmacokinetic monitoring. Patients with renal dysfunction may be spared further kidney damage by avoiding long-term vancomycin therapy, and once-daily treatments decrease hospital costs, including nursing time.
Although vancomycin still has the majority of the MRSA antibiotic hospital market, 33% of surveyed hospitalists report decreasing their vancomycin use over the last 12 months, and this trend is projected to continue in light of new broad-spectrum generic alternatives.26 Daptomycin has been shown to be a cost-effective alternative to vancomycin for gram-positive bloodstream infections requiring IV antibiotic therapy.27 MRSA community-acquired skin and soft tissue infections with MRSA bacteremia is another niche for daptomycin.28,29 Hospira may launch the first generic formulation of daptomycin in 2016, since multiple patent lawsuits from Cubist Pharmaceuticals were ruled invalid.
Tigecycline (Tygacil) is an IV antibiotic that is restricted on most hospital formularies. An alternative in polymicrobial infections—except diabetic foot infections—it is associated with adverse effects that include an increase in all-cause mortality, according to a meta-analysis.28 A black box warning was added to the package insert stating that tigecycline use should be reserved for situations when alternative treatments are not suitable.30 A generic formulation is expected to be available in September 2016.
Emtricitabine, efavirenz, and tenofovir (Atripla): Atripla is a brand-name drug consisting of emtrici-tabine, efavirenz, and tenofovir. This combination decreases the pill burden of HIV treatment at a cost of 40% more than alternative regimens with similar efficacy (e.g., generic lamivudine [currently available], generic efavirenz [expected in 2016], and brand-name tenofovir [generic expected in 2017]).31,32 Atripla is patent-protected until 2018.
HIV care costs more in the U.S. than any other country in the world. The U.S. spent $15 billion for care of patients with HIV in 2012.31 Switching patients to generic versions of these medications is expected to save the country over $1 billion.32 By 2022, it is estimated that HIV will cost $200 per year to treat, 100 times less than today’s estimates of $20,000 per patient per year.31 Generic equivalents of the HIV drugs abacavir, abacavir/lamivudine, and abacavir/lamivudine/zidovudine are all currently available.
Cardiovascular Medications
Hypertension, hyperlipidemia, and diabetes are three of the most common disease states requiring pharmacotherapy, and in many cases, polypharmacy. These disease states have a significant impact on healthcare expenditures worldwide. Pharmacy claims data from Australia revealed a tremendous cost-savings opportunity when using generic-equivalent medications for these three disease states. Had generic therapeutic equivalents been prescribed for branded drugs, the Australian healthcare system could have avoided 18% of their annual healthcare expenditures, approaching US $72 million.33 In addition, observational studies suggest that generic statin use is associated with increased adherence, yielding optimal outcomes in patients aged >65 years.34
Olmesartan (Benicar), olmesartan and amlodipine (Azor), and olmesartan HCT (Benicar HCT): Compliance and persistence for ACE inhibitors and angiotensin II receptor blockers were evaluated using a prescription database of 50,000 users. Compliance, persistence, and switching behavior did not vary among angiotensin II receptor blockers, supporting the prescribing of generic, inexpensive angiotensin II receptor blockers as they become available.35 Olmesartan, olmesartan and amlodipine, and olmesartan HCT generics are expected to be available in October 2016.
Dipyridamole (Aggrenox) bioavailability is adversely affected by increased gastric pH. The buffered extended-release formulation that includes tartaric acid and low-dose aspirin improves bioavailability in the presence of elevated gastric pH.36 The generic formulation is expected to be available in January 2017.
Rosuvastatin (Crestor) is AstraZeneca’s top pharmaceutical product. Rosuvastatin is a first-line hyperlipidemia therapy and is considered the most potent statin. In 2015, AstraZeneca earned $5 billion in revenue from Crestor.37 Although the patent expired in March 2016, a 6-month extension was granted to AstraZeneca under the pediatric trials extension program. Any sales of generic rosuvastatin prior to July 8 will incur a 39% royalty fee to AstraZeneca. The first generic rosuvastatin became available in May 2016.
Ezetimibe (Zetia): When ezetimibe was added to statin therapy, LDL cholesterol was decreased more than with statin therapy alone.38 The first AB-rated ezetimibe generic from Glenmark is expected to be available in December 2016. Simvastatin/ezetimibe generic is expected in 2017.
Antineoplastic Medication
Imatinib (Gleevec): Bioequivalence of imatinib generic was confirmed in adult patients with chronic myeloid leukemia.39 The acquisition cost per tablet is approximately $300. The generic became available in February in the U.S. (see Table 2). There is approximately a $30 cost savings per tablet for the generic version as of May 2016.
Neurologic Disorder Medications
Quetiapine (Seroquel) generated $1.2 billion for Astra Zeneca in 2015.40 Quetiapine is currently available as a multisource generic. Quetiapine extended-release tablet is marketed as better tolerated than the immediate-release product and is expected to be available in November 2016.
Armodafinil (Nuvigil) has demonstrated similar efficacy to modafinil. Since modafinil is already available as a multisource generic, the acquisition cost per tablet is $5 versus $20 for Nuvigil.17 Should the price of generic modafinil become competitive, armodafinil may be a candidate for hospital therapeutic interchange programs. The first generic for armodafinil is expected in June 2016.
Atomoxetine (Strattera) is a novel norepinephrine reuptake inhibitor that has a niche market for ADHD in patients who cannot tolerate stimulants such as methylphenidate or mixed amphetamine salts, or those who do not want to be prescribed a C-2 controlled substance. Both stimulants are available generically for about $1 per tablet, but the branded extended-release formulations of both stimulants range from $5 to $10 per tablet.17 Atomoxetine’s market share for ADHD in adults and children will increase as the price becomes more competitive, especially since atomoxetine is a legend drug with no abuse potential. The first generic for atomoxetine is expected in May 2017.
Eletriptan (Relpax) is one of six serotonin-receptor antagonists indicated for migraine treatment. Some advantages for eletriptan in this drug class include both efficacy and potency versus the innovator’s product, sumatriptan.41 Disadvantages include more side effects and clinically significant drug interactions than competitor’s drugs. Availability is expected in late 2016.
Urology Medications
Tolterodine (Detrol), solifenacin (Vesicare): Although underdiagnosed and often untreated, overactive bladder is estimated to affect one in six Americans >40 years of age.42 Tolterodine and solifenacin are the pharmaceutical market leaders at present, and both are available generically in the U.S.
Darifenacin (Enablex): The extended-release patent extension for Warner Chilcott’s Enablex was extended to 2015, delaying marketing of a generic. Darifenacin extended-release has an FDA-approved AB-rated generic from Anchen Pharmaceuticals, but it is not yet available.20 Generic immediate-release formulations are available from Anchen and Par.
Conclusion
Market exclusivity for branded prescription drugs is necessary to incentivize research and development in the pharmaceutical industry; however, there is a delicate balance between incentivizing research and development for new innovator drug therapies and ensuring affordable healthcare for all. Multisource generic medications are more affordable for patients and help decrease healthcare costs. If there is no competition in the generic marketplace for a needed generic drug, the generic drug manufacturer can raise the price several thousand percent or more. Additional legislation may be needed to promote competition in the generic marketplace to control drug costs.
REFERENCES
1. Chance-Ortiz M. The global outlook for generics, biosimilar and API manufacturers: trends, opportunities, and challenges. Pharmaceutical Outsourcing, January 31, 2016. www.pharmoutsourcing.com/Featured-Articles/182577-The-Global-Outlook-for-Generics-Biosimilar-and-API-Manufacturers-Trends-Opportunities-Challenges. Accessed March 31, 2016.
2. Dylst P, Vulto A, Godman B, et al. Generic medicines: solutions for a sustainable drug market? Appl Health Econ Health Policy. 2013;11(5):437-443.
3. Vogler S. The impact of pharmaceutical pricing and reimbursement policies on generics uptake: implementation of policy options on generics in 29 European countries—an overview. GaBI J. 2012;1(2):93-100.
4. Godman B, Bennie M, Baumgartel C, et al. Essential to increase the use of generics in Europe to maintain comprehensive health care. Farmeconomia Health Econ Ther Pathw. 2012;13(3):5-20.
5. Worth T. The rising cost of generic drugs. Med Econ. 2015;92(5):36-39.
6. Traynor K. Drug price hikes and shortages have similar roots, experts say. AJHP. 2016;73:98-99.
7. Wiske C, Ogbechie O, Schulman K. Options to promote competitive generics markets in the United States. JAMA. 2015;314(20):2129-2130.
8. Hatch-Waxman Act, Pub. L. No. 98-417, tit I. § 101, 98 Stat. 1585 (1984 codified as amended at 21 USC § 355 (2012 & Supp. I 2013).
9. Patient Protection and Affordable Care Act, Pub. L. No. 111-148, 124 Stat. 119 (2010) (codified as amended in scattered sections of 26 and 42 U.S.C.).
10. Biologics Price Competition and Innovation Act of 2009, Pub. L. No. 111-148, tit. VII, §§ 7001-7003, 124 Stat. 804 (codified as amended at 21 U.S.C. §§ 355, 355c and 42 U.S.C. § 262).
11. Paradise J. The legal and regulatory status of biosimilars: how product naming and state substitution laws may impact the United States healthcare system. Am J Law Med 2015;41:49-84.
12. Robison C. Biosimilars: progress and perspectives for managed care. Am J Managed Care. 2015;20(s):8-11. https://ajmc.s3.amazonaws.com/_media/_pdf/FDAWinter12.2015Web.pdf. Accessed March 27, 2015.
13. Blackstone EA, Joseph PF. The economics of biosimilars. Am Health Drug Benefits. 2013;6(8):469-478.
14. Wang B, Liu J, Kesselheim AS. Variations in time of market exclusivity among top-selling prescription drugs in the United States. JAMA Intern Med. 2015:175(4):635-637.
15. Amin T, Kesselheim AS. Secondary patenting of branded pharmaceuticals: a case study of how patents on two HIV drugs could be extended for decades. Health Aff(Millwood). 2012;31(10):2286-2294.
16. Nelson R, McAdam-Marx C, Evans M, et al. Patent extension policy for paediatric indications: an evaluation of the impact within three drug classes in a state Medicaid programme. Appl Health Econ Health Policy. 2011;9(3):171-181.
17. Cardinal Health OrderExpress. https://orderexpress.cardinalhealth.com. Accessed March 31, 2016.
18. Elsevier Clinical Solutions. White paper. The impact of rising generic drug prices on the US drug supply chain. 2015. www.ncpa.co/pdf/elsevier_wp_genericdrug.pdf. Accessed March 31, 2015
19. Serajuddin H, Serajuddin A. Value of pharmaceuticals: ensuring the future of research and development. J Am Pharm Assoc 2006;46:511-516.
20. FDA. Orange Book: approved drug products with therapeutic equivalence evaluations. www.accessdata.fda.gov/scripts/cder/ob/docs/queryai.cfm. Accessed May 9, 2016.
21. Therapeutic Research Center. PL Detail-Document #320553: Anticipated availability of first-time generics. Pharmacist’s Letter/Prescriber’s Letter. May 2016. http://pharmacistsletter.therapeuticresearch.com/pl/ArticlePDF.aspx?s=PL&DetailID=280401. Accessed May 17, 2016.
22. OptumRx pipeline forecast 2014. https://hcp-prod.optumrx.com/vgnlive/HCP/Assets/RxNews/ORX6204_141201_B2B-NEWSLETTER-PipelineReport_FINAL_Q4.pdf. Accessed March 31, 2016.
23. Grabowski H, Kyle M, Mortimer R, et al. Evolving brand-name and generic drug competition may warrant a revision of the Hatch-Waxman Act. Health Aff (Millwood). 2011;30(11):2157-2166.
24. Hao J, Rodriguez-Monguio R, Seoane-Vazquez E. Fixed-dose combination drug approvals, patents and market exclusivities compared to single active ingredient pharmaceuticals. PLoS ONE 2015;10(10):e0140708.
25. Griffith M, Postelnick M, Scheetz M. Antimicrobial stewardship programs: methods of operation and suggested outcomes. Expert Rev Anti Infect Ther. 2012;10(1):63-73.
26. Despite IV vancomycin’s stronghold on the MRSA antibiotic hospital market, surveyed ID specialists report shifting away from IV vancomycin to branded agents, including Pfizer’s Zyvox and Cubist/Novartis’s Cubicin. July 19, 2012. FierceBiotech: The Biotech Industry’s Daily Monitor. www.fiercebiotech.com/press-releases/despite-iv-vancomycin-s-stronghold-mrsa-antibiotic-hospital-market-surveyed. Accessed March 27, 2016.
27. Wilke M. Multiresistant bacteria and current therapy—the economical side of the story. Eur J Med Res. 2010;15(12):571-576. Review. PubMed PMID:21163732; PubMed Central PMCID: PMC3352106.
28. Eckmann C, Dryden M. Treatment of complicated skin and soft-tissue infections caused by resistant bacteria: value of linezolid, tigecycline, daptomycin and vancomycin. Eur J Med Res. 2010;15(12):554-563.
29. Verhoef T, Morris S. Cost-effectiveness and pricing of antibacterial drugs. Chemical Biology & Drug Design. 2015;85(1):4-13.
30. Pfizer, Inc. Tygacil product monograph. January 2016. http://labeling.pfizer.com/showlabeling.aspx?id=491&pagename=tygacil_fly. Accessed May 9, 2016
31. Maxmen A. Generic HIV drugs will widen US treatment net. Nature. 2012;488(7411):267.
32. Nordqvist J. Switching to generic HIV drugs could save the US billions. MNT. Jan 16, 2013. www.medicalnewstoday.com/articles/254972.php. Accessed March 28, 2016.
33. Heinze G, Hronsky M, Reichardt B, et al. Potential savings in prescription drug costs for hypertension, hyperlipidemia, and diabetes mellitus by equivalent drug substitution in Austria: a nationwide cohort study. Appl Health Econ Health Policy. 2015;13(2):193-205.
34. Gagne J, Choudhry N, Kesselheim A, et al. Comparative effectiveness of generic and brand-name statins on patient outcomes: a cohort study. Ann Intern Med. 2014;161(6):400-407.
35. Vegter S, Nguyen N, Visser S et al. Compliance, persistence, and switching patterns for ACE inhibitors and ARBS. Am J Manag Care. 2011;17:609-616.
36. Derendorf H, VanderMaelen C, Brickl R, et al. Dipyridamole bioavailability in subjects with reduced gastric acidity. J Clin Pharmacol. 2005;45(7):845-850.
37. Astra Zeneca revenue from top product Crestor from 2006 to 2015 (in million dollars). Statista- The Statistics Portal. www.statista.com/statistics/266552/astrazenecas-crestor-revenue/. Accessed March 28, 2016.
38. Cannon C, Blazing M, Braunwald E. Ezetimibe plus a statin after acute coronary syndromes. N Engl J Med. 2015;373(15):1476-1477.
39. Arora R, Sharma M, Monif T, Iyer S. A multi-centric bioequivalence trial in Ph+ chronic myeloid leukemia patients to assess bioequivalence and safety evaluation of generic imatinib mesylate 400 mg tablets. Cancer Res Treat. 2016;Feb 12 [Epub ahead of print].
40. AstraZeneca’s revenue from top product Seroquel from 2006 to 2015 (in million U.S. dollars) Statista–The Statistics Portal. www.statista.com/statistics/266551/astrazenecas-revenue-from-top-product-seroquel-since-2006/. Accessed March 28, 2016.
41. Oliver R, Taylor A. Choosing the right triptan: discussion of current triptan options in the treatment of migraine. Pract Pain Manag. 2012. www.practicalpainmanagement.com/pain/headache/migraine/choosing-right-triptan?page=0,1. Accessed March 31, 2016.
42. Global overactive bladder (OAB) therapeutics market to exceed $4 billion by 2017, according to a new report by Global Industry Analysts, Inc. PRWeb. August 25, 2011. www.prweb.com/releases/overactive_bladder/OAB_therapeutics/prweb8585241.htm. Accessed March 31, 2016.
To comment on this article, contact rdavidson@uspharmacist.com.