ABSTRACT: The available antiretroviral (ARV) drugs for the treatment of HIV have expanded since 2018. Novel types of ARVs with new mechanisms of action have been approved, including ibalizumab-uiyk and fostemsavir. There have also been approvals of combination tablets of previously available drugs, such as bictegravir/emtricitabine/tenofovir alafenamide and dolutegravir/lamivudine. Finally, there has been an expansion in approved indications for previously available ARVs themselves, such as emtricitabine/tenofovir alafenamide for use in pre-exposure prophylaxis.
US Pharm. 2020:45(10):17-25.
The mainstays of antiretroviral (ARV) agents for treatment of HIV have largely consisted of the same handful of drug classes for years. HIV treatment was first attempted with nucleoside reverse transcriptase inhibitors (NRTIs), the first FDA-approved class of ARVs. Gradually, more and more classes were discovered and made available for treatment in various, often complex, regimens (TABLE 1).1-3 After the approval of raltegravir in 2007, new HIV drug approvals were largely for repurposed available drugs and new combination drugs. This changed in 2018 with the approval of ibalizumab-uiyk, the first novel ARV in over a decade.4 Ibalizumab-uiyk was quickly followed by a new nonnucleoside reverse transcriptase inhibitor (NNRTI), doravirine, later that year and a novel attachment inhibitor, fostemsavir, in 2020 (TABLE 2).5-7
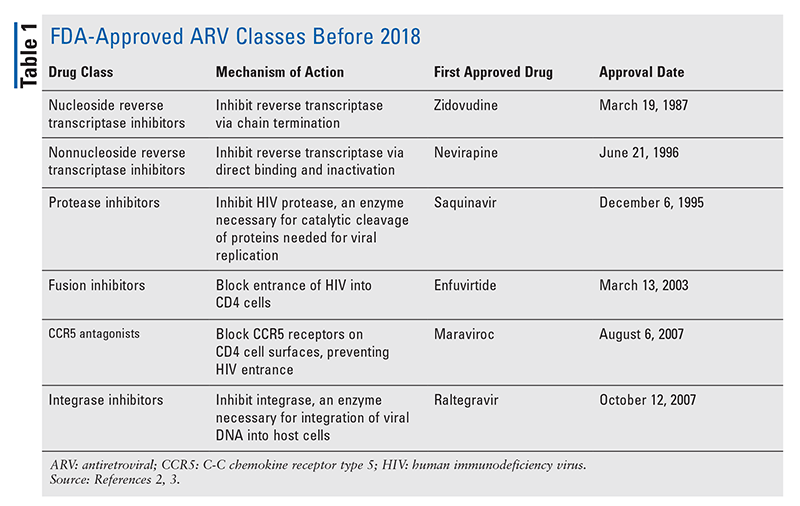
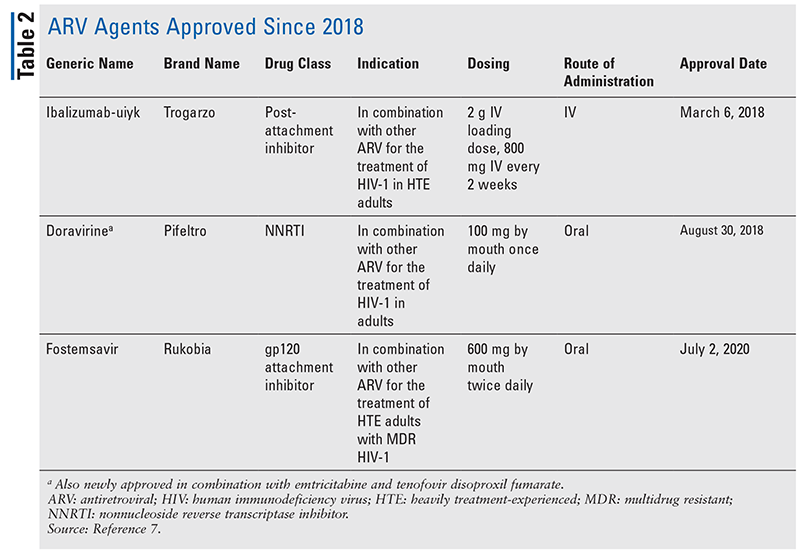
While novel drugs are often the most exciting new drug approvals, ARVs have improved in other ways. Given the enormous pill burden of many HIV regimens, newly approved combination ARVs (TABLE 3) can make life much easier for patients. Use of such agents and limiting the necessary pill burden have demonstrated improved adherence and overall outcomes.8,9 There have also been expansions in approved indications for previously available ARVs (TABLE 4), offering new options for certain patient populations and indications.10-14
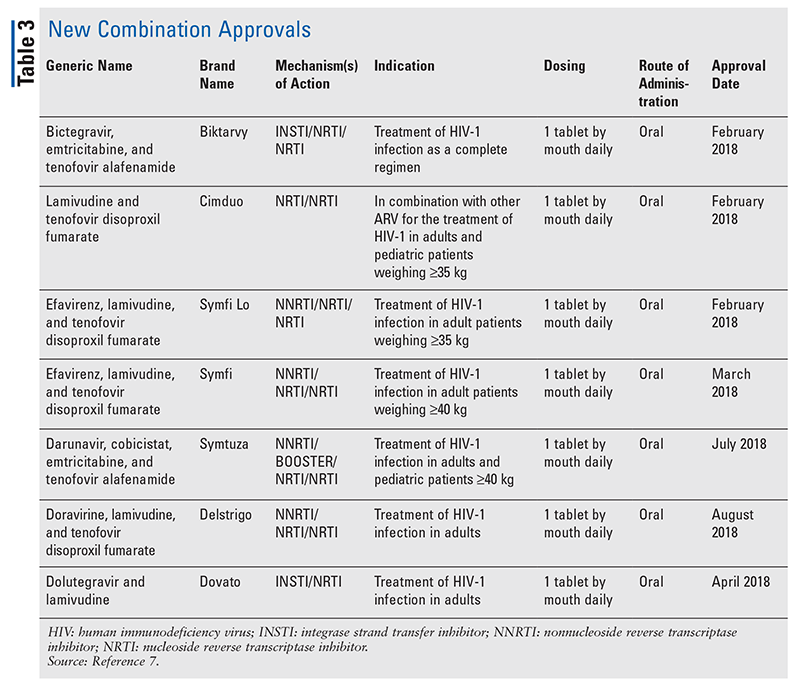
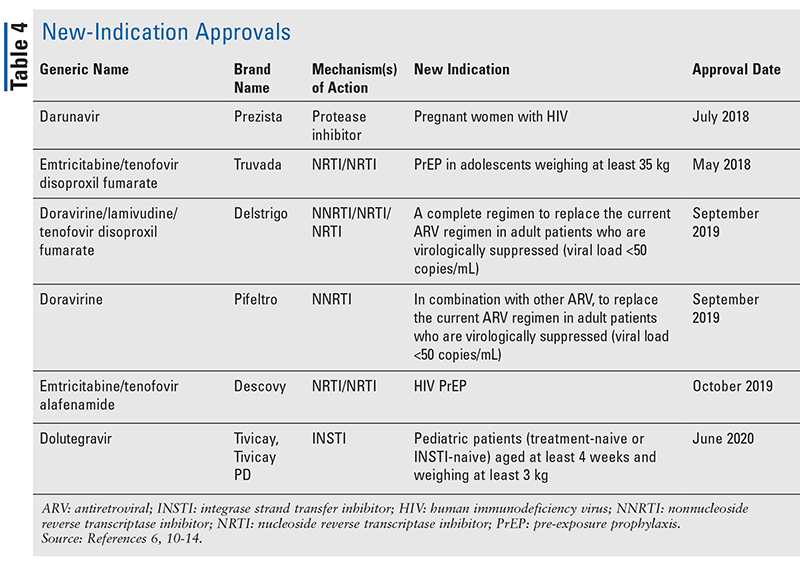
Although there are many new approvals since 2018, this article profiles four of the newly approved medications and one new indication. These approvals were selected to demonstrate a sample of the wide variety of new agents becoming available, as well as emphasize some new options that offer a unique opportunity in the treatment of HIV.
Ibalizumab-Uiyk (Trogarzo)
Ibalizumab-uiyk is an injectable recombinant monoclonal antibody that binds to the surface proteins of CD4 cells leading to conformational changes that prevent the postattachment steps required for HIV-1 fusion and entry into the cell. It is the only monoclonal medication used in the treatment of HIV. Because of its unique binding specificity, ibalizumab-uiyk blocks viral entry without causing immunosuppression, a concern in many monoclonal medications.4,15 It is indicated in combination with other ARVs for treatment in heavily treatment-experienced (HTE) adults with multidrug resistant (MDR) HIV-1 who are failing their current ARV therapy regimen.4
The approval of ibalizumab-uiyk was based on a trial conducted on 40 HTE HIV patients with viral load greater than 1,000 copies/mL and a documented resistance to at least one NRTI, NNRTI, and a protease inhibitor. Patients were also required to have had at least 6 months of treatment and to have recently failed therapy. The trial included three periods, beginning with a 6-day period where baseline viral load was established and confirmed (control period). On day 7, patients received a 2,000-mg loading dose of ibalizumab-uiyk in addition to their standard ART regimen (functional monotherapy period). At day 14, viral load was reassessed and regimens were monitored and adjusted to ensure proper activity against their HIV infection. Ibalizumab-uiyk 800 mg was administered IV every 2 weeks until conclusion of the study (maintenance period). The primary endpoint evaluated the difference in the proportion of viral load decrease from the control period to the functional monotherapy period, specifically comparing the percentage of patients with a ³0.5 log10 decrease in overall viral load. During the control period, 3% of patients were found to have such a decrease, while the functional monotherapy period resulted in 83% of patients meeting the stated viral-load decrease. At the end of 25 weeks, 43% of patients achieved a viral load of <50 copies/mL.16
Based on this study, the approved dosing of ibalizumab-uiyk is a one-time IV loading dose of 2,000 mg, followed by an 800-mg IV maintenance dose every 2 weeks.4,16 Careful monitoring is required for 1 hour after administration of the first infusion for infusion-related reactions, with subsequent infusion monitoring reduced to 15 minutes after toleration of the initial dose.4 Ibalizumab-uiyk seems to be otherwise well-tolerated; the most adverse effects associated with ibalizumab-uiyk were nausea, dizziness, and diarrhea.4,16
Fostemsavir (Rukobia)
Fostemsavir is a novel ARV indicated for combination therapy in HTE adults with known MDR HIV-1, specifically for patients who are failing current ART due to potential resistance, intolerance, or safety considerations.4 It is the first FDA-approved attachment inhibitor. After enzymatic activation to the active molecule temsavir, it binds to gp120, a viral envelope glycoprotein necessary for viral attachment to CD4 cells. This prevents viral entry into CD4 cells, effectively stopping viral replication. Because of this novel mechanism, fostemsavir is unlikely to induce resistance to itself through the selective pressure associated with most other ARVs.6,17
Fostemsavir was evaluated for both safety and efficacy in a randomized, double-blinded, placebo-controlled clinical trial (BRIGHTE) including 371 HTE HIV-1 patients.16,17 Patients in the main cohort were treated with both their baseline ART regimen together with either fostemsavir 600 mg twice daily or placebo for a total of 8 days. Upon assessment on day 8, fostemsavir patients demonstrated significantly lower serum levels of viral HIV-RNA. After this initial treatment period, all patients were transitioned to fostemsavir 600 mg twice daily. At the 24-week mark, 53% of study participants achieved effective undetectable levels of viral suppression, with suppression improving to 60% at week 96.17,18
The most commonly reported adverse effect from fostemsavir was nausea. More severe reactions including elevations in liver enzymes were reported in patients with hepatitis B or C coinfection.17,18 Other studies reported minor incidence of headache, rash, and diarrhea. The active metabolite temsavir is metabolized by CYP3A4, so strong inducers such as carbamazepine, rifampin, and St. John’s wort should be avoided to prevent decreased serum temsavir concentration.17-19
Bictegravir/Emtricitabine/Tenofovir
Alafenamide (Biktarvy)
Bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF) is a fixed-dose, once-daily combination tablet containing a novel integrase inhibitor and two nucleoside/nucleotide reverse transcriptase inhibitors. The individual components in Biktarvy are bictegravir 50 mg (an integrase strand transfer inhibitor [INSTI]), emtricitabine 200 mg (an NRTI) and tenofovir alafenamide 25 mg (an NRTI). BIC/FTC/TAF is indicated as a complete regimen in HIV-1–infected adults with no history of ART or to replace the current ART regimen of patients who are virologically suppressed (viral load £50 copies per mL) on a stable ARV regimen for at least 3 months and no history of treatment failure or resistance to its individual components. Most significantly, it is a one-pill, complete regimen requiring no other ARVs.20
BIC/FTC/TAF was approved in 2018 after four clinical trials. Two of the clinical trials tested the efficacy and safety of BIC/FTC/TAF in patients with no history of ART: Trial 1489 (randomized, BIC/FTC/TAF vs. abacavir/ dolutegravir/lamivudine, [ABC/DTG/3TC] and Trial 1490 (randomized, BIC/FTC/TAF vs. dolutegravir + emtricitabine/tenofovir alafenamide [DTG + FTC/TAF]).21,22 In Trial 1489, the primary endpoint of viral load <50 copies/mL was achieved in 92% of the BIC/FTC/TAF group and 93% of the ABC/DTG/3TC group (treatment difference of -0.6%; 95% confidence interval [CI], -4.8% to 3.6%). In Trial 1490, the primary endpoint of viral load <50 copies/mL was achieved in 89% of the BIC/FTC/TAF group and 93% of the DTG + FTC/TAF group (treatment difference of -3.5%; 95% CI, -7.9% to 1.0%).21,22
The other two trials tested efficacy and safety of BIC/FTC/TAF in virologically suppressed patients who would be switched from their current ART regimens to BIC/FTC/TAF: Trial 1844 (randomized, switching from dolutegravir plus abacavir/lamivudine [DTG + ABC/3TC] or ABC/DTG/3TC to BIC/FTC/TAF) and Trial 1878 (open-label, switching from atazanavir [ATV] or boosted darunavir plus abacavir/emtricitabine [DRV + ABC/3TC] or emtricitabine/tenofovir disoproxil fumarate [FTC/TDF] to BIC/FTC/TAF).23,24 In Trial 1844, the primary endpoint of patients who lost their virologic suppression status (viral load ³50 copies per mL) was seen in 1% of the BIC/FTC/TAF group and <1% of the original regimen group (treatment difference of 0.7%, 95% CI, -1.0% to 2.8%). In Trial 1878, the primary endpoint of patients who lost their virologic suppression status was seen in 2% of the BIC/FTC/TAF group and 2% of the original regimen group (treatment difference of 0.0%; 95% CI, -2.5% to 2.5%).23,24
BIC/FTC/TAF is dosed once daily by mouth with or without food. The most commonly reported adverse reactions seen in patients were diarrhea, nausea, and headache.20-24
Dolutegravir/Lamivudine (Dovato)
Combination dolutegravir/lamivudine (DTG/3TC), an INSTI and NRTI, was approved in 2019. It is the first two-drug, fixed-dose complete regimen for the treatment of HIV-1 infection in treatment-naive adult patients. This contrasts with the traditionally required three-drug standard-of-care regimen options. This may offer opportunity in patients who cannot tolerate any of the more common three-drug regimen due to adverse effects or unavoidable drug interactions.25,26
The efficacy and safety of combination dolutegravir/lamivudine were demonstrated in two identical studies, GEMINI-1 and GEMINI-2.27 These studies enrolled a total of 1,433 HIV-1–infected, treatment-naive adults who were randomized to receive a two-drug regimen, dolutegravir 50 mg plus lamivudine 150 mg, or a three-drug regimen, dolutegravir plus emtricitabine plus tenofovir disoproxil fumarate. The primary endpoint of achieving a viral load <50 copies/mL showed DTG/3TC to be noninferior—91% of the 716 two-drug patients and 93% of the 717 three-drug patients (treatment difference of -1.7%; 95% CI, -4.4% to 1.1%)—when the results of the two studies were pooled together. The pooled studies also showed that, numerically, fewer patients had drug-related adverse events in the two-drug regimen as opposed to the three-drug regimen (126 of 716 [18%] and 169 of 717 [24%], respectively). Due to noninferior efficacy and a similar tolerability profile, the Department of Health and Human Services was able to include fixed-dose combination DTG/3TC in the HIV/AIDS guidelines as a recommended initial regimen for most people with HIV.27
Combination dolutegravir/lamivudine is dosed once daily by mouth with or without food. The most common adverse reactions seen in patients are headache, nausea, diarrhea, fatigue, and insomnia. Despite being a two-medication combination tablet, there is no need to administer it with other ARV medications because it is a complete therapy regimen.25-27
Emtricitabine/Tenofovir Alafenamide
for Pre-exposure Prophylaxis (PrEP)
Since 2012, the only FDA-approved option for PrEP has been FTC/ TDF.11 This changed in 2019 with the approval of combination emtricitabine/tenofovir alafenamide (FTC/TAF),13 a structurally similar combination medication. It is now indicated for use in PrEP in uninfected patients at high risk for HIV transmission via sexual intercourse. This excludes patients at risk from receptive vaginal intercourse, as this population has not yet been evaluated.
Approval of PrEP is based on the recent DISCOVER trial, a randomized, double-blind study comparing FTC/TAF with standard-of-care FTC/TDF in reducing the risk of acquiring HIV-1 infection in men and transgender women who have sex with men and are at risk of HIV-1 infection.7 The trial was conducted as a randomized, double-blind, parallel-group study with a minimum follow-up of 48 weeks, with at least 50% of the participants having 96 weeks of follow-up after randomization. It enrolled 5,399 participants who were randomized 1:1 into either group. The study’s primary objective was to assess the incidence of HIV-1 infection per 100 person-years. Among the 2,694 participants in the FTC/TAF arm, HIV-1 incidence was seen in 7 patients (0.16/100 person-years). Among the 2,693 participants in the FTC/TDF arm, HIV-1 incidence was seen in 15 patients (0.34/100 person-years). These incidences demonstrate that FTC/TAF was noninferior to FTC/TDF in study participants who were at risk of acquiring HIV and, ultimately, allowed the FDA to add PrEP to the indications for FTC/TAF.13
The place of FTC/TAF in HIV therapy is likely still unclear. While it has found use in other areas of HIV treatment, its benefit over the alternative in this setting is yet to be conclusively determined. While similar, the TAF may offer a lower incidence of bone-density changes and renal dysfunction, adverse effects associated with TDF. This difference is not well established, however, with some data suggesting that, in regimens that do not include a pharmacokinetic booster such as cobicistat or ritonavir, this benefit may not be clinically significant. 27-29
Conclusion
Due to the complexity of HIV and its potential for resistance, ARV therapy can quickly become burdensome for patients. Between 2018 and the present, the medical community has seen a wide expansion of FDA-approved ARV therapies for the treatment of HIV. These therapies include new fixed-dose, combination tablets; new drugs that work through different mechanisms than those previously on the market; and even new indications for previously existing medications. These new medications have already made an impact on current treatment guidelines and may help patients live longer, healthier lives.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
1. Vella S, Schwartländer B, Sow SP, et al. The history of antiretroviral therapy and of its implementation in resource-limited areas of the world. AIDS. 2012;26(10):1231-1241.
2. Arts EJ, Hazuda DJ. HIV-1 antiretroviral drug therapy. Cold Spring Harb Perspect Med. 2012;2(4):a007161.
3. Chaudhuri S, Symons JA, Deval J. Innovation and trends in the development and approval of antiviral medicines: 1987–2017 and beyond. Antiviral Res. 2018;155:76-88.
4. Trogarzo (ibalizumab-uiyk) package insert. Montreal, Quebec: Theratechnologies Inc; March 2018.
5. Pifeltro (doravirine) package insert. Whitehouse Station, NJ: Merck & Co, Inc; March 2019.
6. Rukobia (fostemsavir) package insert. Research Triangle Park, NC: GlaxoSmithKline; July 2020.
7. Pan F, Chernew ME, Fendrick AM. Impact of fixed-dose combination drugs on adherence to prescription medications. J Gen Intern Med. 2008;23(5):611-614.
8. Verma AA, Khuu W, Tadrous M,et al. Fixed-dose combination antihypertensive medications, adherence, and clinical outcomes: a population-based retrospective cohort study. PLoS Med. 2018;15(6):e1002584.
9. National Institutes of Health. HIVinfo. FDA-approved HIV medicines. hivinfo.nih.gov/understanding-hiv/fact-sheets/fda-approved-hiv-medicines. Accessed September 22, 2020.
10. Prezista (darunavir) package insert. Titusville, NJ: Janssen Therapeutics; May 2019.
11. Truvada (emtricitabine/tenofovir disoproxil fumarate) package insert. Foster City, CA: Gilead Sciences, Inc; June 2004/2020.
12. Delstrigo (doravirine/lamivudine/tenofovir disproxil fumerate) package insert. White House Starion, NJ: Merck & Co, Inc; August 2018.
13. Descovy (emtricitabine/tenofovir alafenamide) package insert. Foster City, CA: Gilead Sciences, Inc; December 2019.
14. Tivicay (dolutegravir) package insert. Research Triangle Park, NC. GlaxoSmithKline; June 2020.
15. Iacob SA, Iacob DG. Ibalizumab targeting CD4 receptors, an emerging molecule in HIV therapy. Front Microbiol. 2017;8:2323.
16. Emu B, Fessel J, Schrader S, et al. Phase 3 study of ibalizumab for multidrug-resistant HIV-1. N Engl J Med. 2018;379:645-654.
17. Li Z, Zhou N, Sun Y, et al. Activity of the HIV-1 attachment inhibitor BMS-626529, the active component of the prodrug BMS-663068, against CD4-independent viruses and HIV-1 envelopes resistant to other entry inhibitors. Antimicrob Agents Chemother. 2013:57(9):4172-4180.
18. Kozal M, Aberg J, Pialoux G. Fostemsavir in adults with multidrug-resistant HIV-1 infection. N Engl J Med. 2020;382(13):1232-1243.
19. Cahn P, Fink V, Patterson P. Fostemsavir: a new CD4 attachment inhibitor. Curr Opin HIV AIDS. 2018;13(4):341-345.
20. Biktarvy (bictecgravir/emtricitabine/tenofovir alafenamide) package insert. Foster City, CA: Gilead Sceinces, Inc; February 2018.
21. Gallant J, Lazzarin A, Mills M, et al. Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380-1489): a double-blind, multicentre, phase 3, randomised controlled non-inferiority trial. Lancet. 2017;390(10107):2063-2072.
22. Sax PE, Pozniak A, Montes M, et al. Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380-1490): a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet. 2017;390(10107):2073-2082.
23. Malina JM, Ward D, Brar I, et al. Switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from dolutegravir plus abacavir andlamivudine in virologically suppressed adults with HIV-1: 48 week results of a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet HIV. 2018;5(7):E357-E365.
24. Daar ES, De Jesus E, Ruane P, et al. Efficacy and safety of switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from boosted protease inhibitor-based regimens in virologically suppressed adults with HIV-1: 48-week results of a randomised, open-label, multicentre, phase 3, non-inferiority trial. Lancet HIV. 2018;5(7):e347-e356.
25. Dovato (dolutegravir/lamivudine) package insert. Research Triangle Park, NC: GlaxoSmithKline. March 2020.
26. Scott LJ. Dolutegravir/lamivudine single-tablet regimen: a review in HIV-1 infection. Drugs. 2020;80(1):61-72.
27. Cahn P, Madero JS, Arribas JR, et al. GEMINI Study Team. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet. 2019;393(10167):143-155.
28. Mayer KH, Molina JM, Thompson MA, et al. Emtricitabine and tenofovir alafenamide vs. emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): primary results from a randomised, double-blind multicentre, active-controlled, phase 3 non-inferiority trial. Lancet. 2020;396(10246):239-254.
29. Hill A, Hughes SL, Gotham D, Pozniak AL. Tenofovir alafenamide versus tenofovir disoproxil fumarate: is there a true difference in efficacy and safety? J Virus Erad. 2018;4(2):72-79.
To comment on this article, contact rdavidson@uspharmacist.com.





