US Pharm.
2006;5(Student suppl):8-9.
Characterized by increased
levels of blood glucose, diabetes mellitus (DM) results from defects in
insulin production, insulin action, or both and is classified based upon the
underlying cause.1 Type 1 DM, also known as insulin-dependent DM or juvenile-onset
diabetes, accounts for approximately 5% to 10% of all cases. The majority of
diagnosed cases are type 2 DM, commonly referred to as non–insulin-dependent DM
or adult-onset diabetes. This form of diabetes may be associated with older
age, obesity, genetics, history of gestational diabetes, impaired glucose
tolerance, physical inactivity, and/or ethnicity. Studies demonstrate that
African-Americans, Hispanic-Americans, American Indians, and some Pacific
Islanders are at a higher risk for diabetes and that adolescents are
increasingly affected by diabetes as a result of obesity.1,2 Also,
during pregnancy, 2% to 5% of women are diagnosed with gestational diabetes,
after which they have a 40% chance of developing diabetes within five years.1
Symptoms of diabetes typically include poly uria, polydipsia, polyphagia,
weight loss, or blurred vision.2,3 Diagnostic guidelines for
diabetes are listed in table 1.

Glycemic Control
Research
concludes that improved glycemic control is ultimately beneficial for both
types of diabetes. In general, for a 1% reduction in hemoglobin A1C, the risk
of developing microvascular complications (e.g., hypertension, stroke,
neuropathy, kidney disease) is reduced by about 40%.2 Early
detection and treatment of diabetes may also decrease the risk of severe
vision loss by 50% to 60% and kidney failure by 30% to 70%.3
Additionally, it may alleviate symptoms of diabetes-induced neuropathy.3
This explains the annual global cost for glucose testing, $3.7 billion, with a
growth rate of 12% to 18%.3
Currently, diabetic patients
must frequently monitor their glucose levels by pricking their fingertip to
draw a drop of blood. Unfortunately, the invasive nature of this procedure
often results in patient complaints and noncompliance. With this in mind,
several companies have created noninvasive glucose monitors. Among this new
class is SpectRx's Real-Time Glucose-Sensing System (RTGSS), as seen in figure
1.
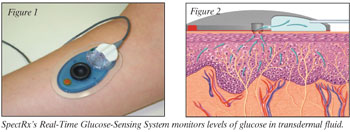
Real-Time Glucose-Sensing System
The RTGSS
measures glucose from transdermal fluid (TDF) using a subcutaneous sensor (figure
2) over a 24- to 72-hour period.4,5 The TDF collection procedure
involves the application of a focused laser beam to the arm or abdomen
(preferable sites) to produce micropores in the stratum corneum of less than
100 micrometers in diameter. A small, flexible disk containing an
energy-absorbing dye is aligned with a handheld laser that produces pulsed
laser energy over three-second intervals. Once the micropores have been
created, the site is covered with a single-use TDF harvesting patch attached
to a continuous vacuum that attracts TDF to the glucose sensor. The vacuum
generates a continuous flow of about 5 to 15 mcL/min, and any excess fluid is
stored in a waste depot within the patch. Since the sensor is external, it
insulates the RTGSS from the body's natural immune response. Patient
instructions are found in table 2.

Measurement of glucose from interstitial fluid reportedly produces glucose concentrations similar to venous blood or plasma following an oral glucose challenge. Since the glucose content of TDF is comparable to that of plasma glucose, it may serve as a surrogate for blood glucose.6
A double-blinded clinical
trial examined the efficacy of the RTGSS. Its results were subsequently
published in Diabetes Technology and Therapeutics in June 2005.4
The study's subjects included various ethnic and age-groups and both sexes. A
small number of participants did not have diabetes and served as a control
group. The
Conclusion
Although
currently unavailable for purchase, transdermal measurement of glucose shows
great promise as a noninvasive method for monitoring. This may increase
patient compliance and decrease costs associated with uncontrolled glucose.
For more information, see SpectRx's Web site (www.spectrx.com) or call (770)
242-8723.
REFERENCES
1. DiPiro T,
Talbert R, Yee G, et al, eds. Pharmacotherapy: A Pathophysiologic Approach.
5th ed.
2. American Diabetes
Association. Diabetes facts and figures. Available at:
www.diabetes.org/diabetes-statistics/national-diabetes-fact-sheet.jsp.
3. CDC. Preventing
diabetes complications. Available at:
www.cdc.gov/diabetes/pubs/general.htm#top. Accessed August 18, 2005.
4. Burdick J, Chase
P, Faupel M, et al. Real-time glucose sensing using transdermal fluid under
continuous vacuum pressure in children with type 1 diabetes. Diabetes
Technol Ther. 2005;7:448-455.
5. SpectRx Inc. The
real-time glucose-sensing system. Available at: www.spectrx.com.
6. Gebhart S, Faupel
M, Fowler R, et al. Glucose sensing in transdermal body fluid collected under
continuous vacuum pressure via micropores in the stratum corneum. SpectRx
Publication, 2002.
To comment on this article,
contact editor@uspharmacist.com.





