US Pharm. 2020;45(9):48-CV3.
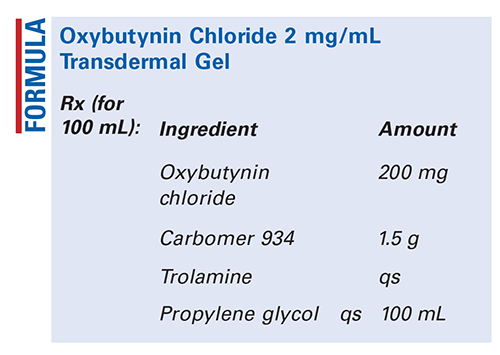
Method of Preparation: Calculate the required quantity of each ingredient for the total amount to be prepared. Accurately weigh or measure each ingredient. Mix the oxybutynin chloride with about 95 mL of the propylene glycol. Add the carbomer 934 and mix well. Dropwise, add the trolamine to the desired viscosity and mix well. Add sufficient propylene glycol to final volume and mix well. Package and label.
Use: Oxybutynin chloride may be used to treat urinary urgency and urinary incontinence.
Packaging: Package in tight, light-resistant containers.
Labeling: Keep out of reach of children. Discard after ____ [time period].
Stability: A beyond-use date of 30 days may be used for this preparation.1
Quality Control: Quality-control assessment can include theoretical weight compared with actual weight, specific gravity, active drug assay, color, clarity, texture–surface, texture–spatula spread, appearance, feel, rheologic properties, and physical observations.2-4
Discussion: The term urinary incontinence refers to the inability to control the passage of urine, and it can range from an occasional leakage of urine to a complete inability to hold any urine. Although it can happen to anyone, incontinence occurs more commonly in women. There are three types of urinary incontinence: stress, urge, and mixed. Stress incontinence occurs during certain activities, such as coughing, sneezing, laughing, or exercise; urge incontinence is characterized by a strong, sudden need to urinate followed by instant contraction of the bladder and involuntary loss of urine; and mixed incontinence includes components of both stress incontinence and urge incontinence.
Oxybutynin chloride (Ditropan, C22H31NO3.HCl, MW 393.95) occurs as a white, crystalline, practically odorless powder. It is freely soluble in water and in alcohol. Oxybutynin chloride is a urinary antispasmodic agent used in the treatment of neurogenic bladder (urgency, frequency, urge incontinence) and uninhibited bladder.1
Carbomer 934 is a synthetic, high-molecular-weight polymer composed of acrylic acid cross-linked with either allyl sucrose or allyl ethers of pentaerythritol. It occurs as a white-colored, fluffy, acidic, hygroscopic powder with a slight characteristic odor. The molecular weight of carbomer 934 is approximately 3 × 106. The pH of a 0.5% to 1.0% dispersion is in the range of 2.5 to 3.5. Carbomer 934 is soluble in water and, after neutralization, in 95% ethanol and glycerin. When carbomers are dispersed in water, an acidic colloidal solution of low viscosity will form that will thicken when an alkaline material, such as triethanolamine, is added. Maximum viscosity can generally be obtained in a pH range of 6 to 11. These gels should be protected from light and should contain an antimicrobial preservative.5
Trolamine (TEA, triethanolamine) is an alkalizing and emulsifying agent. It occurs as a variable mixture of alkanolamines and is a clear, colorless to pale yellow–colored, viscous liquid with a slight ammoniacal odor. Trolamine has a specific gravity of about 1.120 g/mL to 1.128 g/mL, and it has a pH of 10.5 in a 0.1 N aqueous solution. Trolamine is highly hygroscopic, it melts at 20°C to 21°C, and it boils at 335°C. Trolamine is miscible with water, 95% ethanol, methanol, and acetone, and it is soluble in chloroform.6
Propylene glycol (C3H8O2) occurs as a clear, colorless, viscous, practically odorless liquid with a sweet taste somewhat resembling glycerin. It has a specific gravity of 1.038 g/mL and is miscible with acetone, chloroform, 95% ethanol, glycerin, and water. Propylene glycol is not miscible with fixed oils or light mineral oil; it will, however, dissolve some essential oils. Propylene glycol is stable, and it may be mixed with numerous other solvents. Since it is hygroscopic, propylene glycol should be stored in an airtight container and protected from light.7
REFERENCES
1. U.S. Pharmacopeia/National Formulary [current revision]. Rockville, MD: U.S. Pharmacopeial Convention, Inc; August 2020.
2. Allen LV Jr. Standard operating procedure for performing physical quality assessment of ointments/creams/gels. IJPC. 1998;2:308-309.
3. Allen LV Jr. Summary of quality-control testing for sterile and nonsterile compounded preparations, part 1: physical and chemical testing. IJPC. 2019;23(3):211-216.
4. Allen LV Jr. Summary of quality-control testing for sterile and nonsterile compounded preparations, part 2: microbiological testing. IJPC. 2019;23(4):299-303.
5. Draganoiu E, Rajabi-Siahboomi A, Tiwari S. Carbomer. In: Sheskey PJ, Cook WG, Cable CG, eds. Handbook of Pharmaceutical Excipients. 8th ed. London, England: Pharmaceutical Press; 2017:165-170.
6. Goskonda SR. Triethanolamine. In: Sheskey PJ, Cook WG, Cable CG, eds. Handbook of Pharmaceutical Excipients. 8th ed. London, England: Pharmaceutical Press; 2017:994-996.
7. Driver S. Propylene glycol. In: Sheskey PJ, Cook WG, Cable CG, eds. Handbook of Pharmaceutical Excipients. 8th ed. London, England: Pharmaceutical Press; 2017:795-798.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





