US Pharm. 2023;48(3):7-12.
ABSTRACT: Neuropathic pain, which affects an estimated 7% to 8% of the U.S. population, is caused by a lesion or disease of the somatosensory system. Neuropathic pain is broadly categorized as central or peripheral, with most patients experiencing peripheral neuropathic pain (PNP). Trigeminal neuralgia, postherpetic neuralgia, and diabetic neuropathy are the most common causes of PNP. PNP is usually difficult to manage and requires multimodal approaches, including pharmacologic and nonpharmacologic interventions, to effectively achieve pain mitigation and minimize physical and/or psychological consequences. Integrated care involving pharmacists and physical therapists can help ensure a comprehensive approach to pain management in patients with PNP.
An estimated 7% to 8% of the U.S. population have neuropathic pain, and 20% to 25% experience chronic continuous or recurrent pain lasting at least 3 months.1 Neuropathic pain occurs more frequently in women and in persons aged older than 50 years.2 Rates of neuropathic pain are anticipated to rise as the global population ages, increasing the need for healthcare professionals to be aware of appropriate approaches to—and common challenges posed by—its management.3
Overview
Neuropathic pain is caused by a lesion or disease of the somatosensory system, which involves nerves located in the muscles, joints, fascia, and skin.1,4 This system includes mechanoreceptors, thermoreceptors, chemoreceptors, pruriceptors, and nociceptors that send signals to the spinal cord and brain from the nerves affected by perceptions of pressure, touch, temperature, position, movement, vibration, and pain.1,2,4 Positive sensory symptoms include paresthesias (e.g., prickling, tingling, creeping feeling on the skin), whereas negative sensory symptoms involve reduction or loss of sensation (e.g., numbness) in the affected area of the body.1,2 Damage to the somatosensory system can result in persistent or paroxysmal neuropathic pain, which patients may describe as burning, sharp, shooting, or electric-like sensations, as well as increased sensitivity to touch (i.e., hyperalgesia or allodynia).1-3 Patients with neuropathic pain may also report secondary symptoms related to depression, anxiety, sleep, and overall quality of life.3
Neuropathic pain has been broadly classified as central or peripheral, with most patients experiencing peripheral neuropathic pain (PNP).1,3 Central neuropathic pain is caused by a lesion or disease of the spinal cord or brain, such as pain secondary to spinal cord or brain injury, stroke, neurodegenerative diseases (e.g., Parkinson’s disease), or demyelinating diseases (e.g., multiple sclerosis).1-3 PNP is caused by a lesion or disease involving some part of the peripheral nervous system, ranging from the dorsal root ganglion and its connections to the spinal cord to the distal periphery.2,3
PNP has numerous etiologies, including peripheral nerve injury (traumatic or surgical), infections (e.g., HIV, leprosy), painful cervical and lumbar radiculopathy, chemotherapy, immune-mediated conditions (e.g., Guillain-Barré syndrome), familial diseases, exposure to environmental toxins, trigeminal neuralgia, postherpetic neuralgia, and diabetic neuropathy.1,2 Of these, trigeminal neuralgia, postherpetic neuralgia, and diabetic neuropathy are the most common causes of PNP.2,3 The following sections discuss management approaches for PNP, with a focus on trigeminal neuralgia, postherpetic neuralgia, and diabetic neuropathy.
Pharmacologic Management
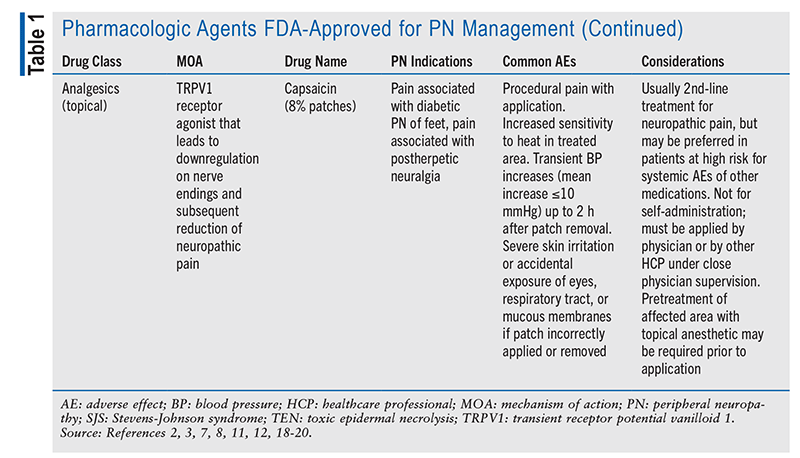
The key treatment goals for PNP are to reduce pain, maintain or improve function, and preserve or improve quality of life.3 Although pharmacotherapy is the foundation of PNP therapy, alleviation of pain is often difficult to achieve.1,3 Therefore, it may be necessary to trial different medications (including combination regimens) in order to determine an effective therapeutic plan with minimal to acceptable adverse effects (TABLE 1).1,2
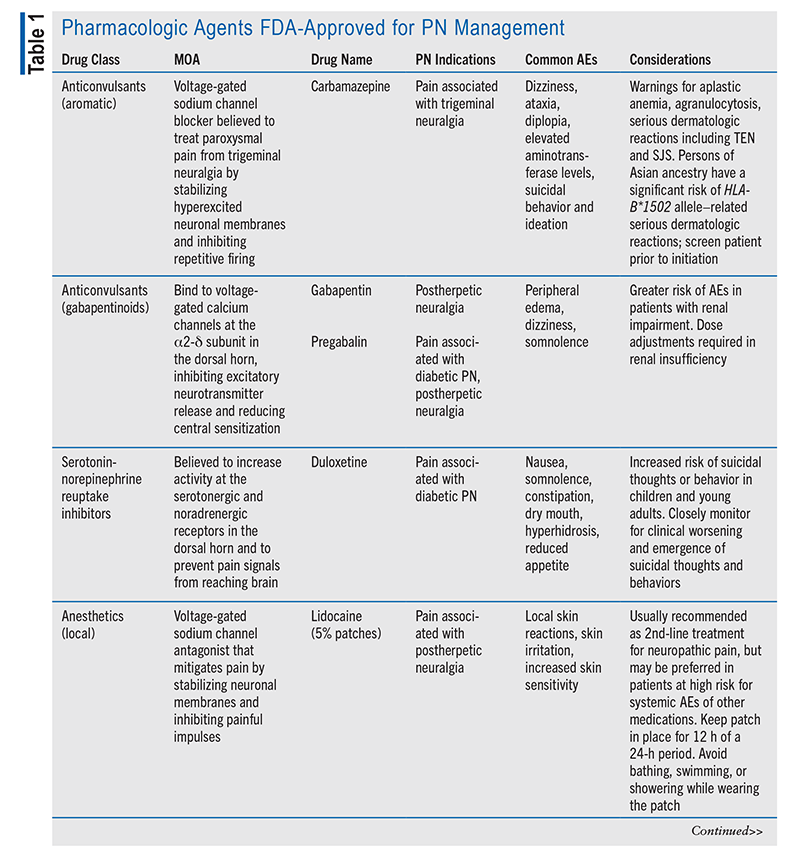
Trigeminal Neuralgia: This chronic orofacial neuropathic pain disorder affects one or more divisions of the trigeminal nerve, which innervates the face.1 Trigeminal neuralgia is characterized by paroxysmal episodes of pain that patients may describe as an electric-like sensation or as shooting or stabbing pain in a region of the face.1,5 These episodes may be severe and intense, last from 1 second to 2 minutes, and are triggered by innocuous stimuli or facial movements (e.g., talking, face washing, chewing, brushing the teeth, touching the face).1,5,6 The pain most frequently occurs on the right side of the face, in the maxillary and mandibular divisions of the trigeminal nerve.5,6 Additionally, although triggered paroxysmal pain is a defining feature of trigeminal neuralgia, up to 50% of patients experience continuous pain between the paroxysmal attacks.5 The continuous pain is often described as a throbbing, aching, or burning.5,6
The most common cause of trigeminal neuralgia is intracranial vascular compression of the trigeminal nerve root; this compression results in the demyelination of large myelinated fibers, which increases their susceptibility to ectopic excitation and discharges.6 Trigeminal neuralgia is more common in women, and the incidence increases with age.5 Because of the severity and frequency of the paroxysmal pain episodes, many people with trigeminal neuralgia report a poor quality of life.5,6 The first line of treatment for trigeminal neuralgia is pharmacologic therapy with a voltage-gated sodium channel blocker such as carbamazepine or oxcarbazepine.5 Patients whose response to pharmacologic therapy is inadequate may require surgical intervention for decompression of the trigeminal nerve.5,6
Postherpetic Neuralgia: Postherpetic neuralgia is a chronic neuropathic pain syndrome caused by herpes zoster (also known as shingles), which is a reactivation of the varicella zoster virus residing in the sensory ganglion.7,8 Herpes zoster, which affects nearly one in three people during his or her lifetime, typically manifests as a painful vesicular maculopapular rash in the dermatome of the affected sensory ganglion.7 The rash is usually unilateral and most commonly appears on the trunk, specifically on the thoracic dermatome. Although the rash tends to resolve within a few weeks, the localized pain may persist for months or years as a result of the peripheral nerve damage that occurred during the acute herpes zoster episode.8 There are several definitions of postherpetic neuralgia; however, it is most often defined as pain that persists for at least 3 months following the onset of a herpes zoster rash.1,8 The three primary types of pain associated with postherpetic neuralgia are ongoing continuous pain not provoked by a stimulus (e.g., continuous burning or throbbing pain), intermittent pain not provoked by a stimulus (e.g., stabbing, electric-like sensation, or shooting pain), and pain related to hyperalgesia or allodynia.7,8
The incidences of both herpes zoster and subsequent postherpetic neuralgia increase with advanced age.7,8 Other risk factors for postherpetic neuralgia include underlying chronic diseases (e.g., diabetes, respiratory disease), severity of the herpes zoster rash, and degree of pain experienced during the acute phase of herpes zoster infection.8 Postherpetic neuralgia may have a tremendous effect on an individual’s quality of life, impacting both physical function and psychological health.7,8 Associated consequences usually include decreased physical activity, anxiety, depression, reduced appetite, and weight loss.7,8 The pain may also lead to sleep disturbances and social isolation resulting from difficulty wearing clothing over the affected area.7
Treatment of postherpetic neuralgia focuses on symptom control via pharmacologic therapy.7,8 The pharmacologic options recommended most often are gabapentinoids, tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, topical analgesics, and local anesthetics.7 Even when evidence-based medications are used, fewer than one-half of patients experience significant reductions in pain (50% or greater).7,8 Therefore, emphasis should be placed on preventative measures to minimize the potential burdens of postherpetic neuralgia on the patient’s quality of life.8 Active immunity in the form of a two-dose series of the recombinant herpes zoster vaccine (Shingrix) is currently recommended for all adults aged 50 years or older, including those who previously received the live zoster vaccine (Zostavax), and for patients aged 18 years or older at increased risk for herpes zoster because of immunodeficiency or immunosuppression.9,10 Randomized, controlled trials have shown that the recombinant vaccine can reduce the risk of herpes zoster by 97% or more in adults older than age 50 years, and in those who develop herpes zoster, the risk of postherpetic neuralgia is reduced nearly 90%.9
Diabetic Neuropathy: Diabetic neuropathy, the most common form of peripheral neuropathy, is the most frequently occurring complication of diabetes (more than 50% of diabetes patients).11,12 Approximately 12% of the U.S. population (and approximately 25% of U.S. adults aged 65 years and older) has diabetes; therefore, diabetic neuropathy can have a significant impact on a large number of people as well as on the U.S. healthcare system.11 Common risk factors for developing diabetic neuropathy include duration of diabetes and increased age.11,12 The most prominent form of diabetic neuropathy is distal symmetric polyneuropathy, which typically presents in a progressive “glove and stocking” pattern.11 With this type of diabetic neuropathy, the patient initially experiences symptoms of sensory loss (e.g., numbness, tingling) and/or weakness of the most distal portion of the fingers and/or toes.11,12 As the neuropathy progresses, the symptoms spread proximally, subsequently affecting the feet, calves, hands, and/or forearms.2,11 More than 40% of patients may also experience burning, electric-like, dull, or sharp pain that worsens during times of stress, fatigue, or lack of sleep.6,12 Painful diabetic neuropathy can significantly impact a patient’s mood, mobility, socialization, and overall quality of life.2,12
Although the exact pathophysiology of diabetic neuropathy has not been fully determined, it is believed to involve metabolic and vascular abnormalities that cause early damage to unmyelinated nerve fibers and progressive damage to myelinated nerve fibers.11 No treatments are available to effectively reverse the nerve damage or prevent the progression of neuropathy; however, glycemic control should be the first step in mitigating diabetic neuropathy.12 Pharmacologic treatment options for the management of pain associated with diabetic neuropathy are similar to those for other types of PNP. Diabetic neuropathy is as difficult to manage as other types of PNP, with limited alternatives for refractory cases.11,12
Nonpharmacologic Approaches
Studies have found that pharmacologic treatment modalities for neuropathic pain are effective in less than 50% of patients.2,13 Therapeutic responses are greatly influenced by expectancy-induced analgesia.2 Therefore, establishing realistic expectations by using a multidisciplinary approach (including pharmacologic and nonpharmacologic interventions) is paramount in the management of PNP.2,3
Nonpharmacologic approaches that integrate various types of physical therapy should be initiated early, in conjunction with pharmacologic treatment, to minimize the impact of the psychological and social consequences of neuropathic pain on the patient.13-15 In PNP management, physical therapy can play a pivotal role in reducing pain via strategies that are focused on functional mobility, gait training, balance, activity tolerance (in addition to exercise), and transcutaneous electrical nerve stimulation (TENS).13,15,16 The primary goals of physical-therapy interventions are to prevent further changes in condition, potentially reverse physical changes (e.g., atrophy, deconditioning), reduce related psychological effects, and minimize the impact on quality of life.13
Physical Therapy: Routine exercise training can provide significant benefits in improving nerve function, lessening neuropathic pain, reducing other sensory dysfunction, and enhancing functional mobility in patients with peripheral neuropathy.16 Exercise training may also be an effective way to lessen some of the secondary consequential symptoms associated with PNP.13,16 Similarly, gait and balance training may help increase a patient’s confidence in community accessibility by improving cadence, stride length, and time spent in single leg stance; direct effects on pain reduction have not been consistently observed, however.16
TENS is an alternative therapy that has been proven effective and is frequently used by physical therapists and physicians to reduce PNP.13,14 It is relatively cost effective and has few contraindications, making it a safe, viable option for PNP therapy.14 Additional strategies for managing pain that are supported by the literature include massage, relaxation techniques, and meditation/mind-body therapy.13,16 Acupuncture and tai chi exercises have also been studied for the treatment of symptoms associated with peripheral neuropathy; however, further research is needed to determine potential benefits for PNP.13,16,17
Patients with PNP should be referred to a local physical therapist for the provision of complementary and comprehensive care for management of PNP.14 Early initiation of physical-therapy modalities may minimize the impact of chronic pain and reduce the economic burden of healthcare expenses related to neuropathic pain.13,14 Providing resources on education, podcasts, and local or virtual support groups is also essential, and this information is available from the Foundation for Peripheral Neuropathy (www.foundationforpn.org). Engaging patients with PNP in a multidisciplinary treatment approach that includes pharmacologic treatment and physical therapy is recommended to achieve optimal outcomes.13-15
The Pharmacist’s Role
Pharmacologic treatment is at the core of PNP therapy. As a result, pharmacists can have a tremendous impact on PNP management. Patients most affected by PNP are older and require careful consideration of their medication regimens because of the increased risk of adverse effects. Many older patients also have comorbidities for which they are taking numerous medications, necessitating medication evaluations and reconciliations performed by pharmacists. In addition, pharmacists must counsel patients on the importance of medication adherence and scheduled dosing regimens in PNP (in contrast to other pain syndromes, which may be treated on an as-needed basis). Finally, pharmacists should offer preventative measures when available and applicable, as in the case of postherpetic neuralgia secondary to herpes zoster infection, which is highly preventable through vaccination.
Conclusion
A multidisciplinary treatment approach for PNP is recommended to achieve optimal patient outcomes. This includes both pharmacologic and nonpharmacologic modalities. Pharmacists can work with physical therapists and patients to initiate integrated and comprehensive therapeutic plans for managing PNP and its negative effects on the patient’s psychological well-being, social interactions, functional mobility, and overall quality of life.
REFERENCES
1. Scholz J, Finnerup NB, Attal N, et al. The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain. 2019;160(1):53-59.
2. Colloca L, Ludman T, Bouhassira D, et al. Neuropathic pain. Nat Rev Dis Primers. 2017;3:17002.
3. Murphy D, Lester D, Clay Smither F, Balakhanlou E. Peripheral neuropathic pain. NeuroRehabilitation. 2020;47(3):265-283.
4. International Association for the Study of Pain. Terminology: pain terms and definitions. www.iasp-pain.org/resources/terminology. Accessed January 31, 2023.
5. Cruccu G, Di Stefano G, Truini A. Trigeminal neuralgia. N Engl J Med. 2020;383(8):754-762.
6. Di Stefano G, Di Lionardo A, Di Pietro G, Truini A. Neuropathic pain related to peripheral neuropathies according to the IASP grading system criteria. Brain Sci. 2020;11(1):1.
7. Schutzer-Weissmann J, Farquhar-Smith P. Post-herpetic neuralgia—a review of current management and future directions. Expert Opin Pharmacother. 2017;18(16):1739-1750.
8. Johnson RW, Rice ASC. Postherpetic neuralgia. N Engl J Med. 2014;371(16):1526-1533.
9. Harbecke R, Cohen JI, Oxman MN. Herpes zoster vaccines. J Infect Dis. 2021;224(12 suppl 2):S429-S442.
10. Shingrix (zoster vaccine recombinant, adjuvanted) product information. Research Triangle Park, NC: GlaxoSmithKline; July 2021.
11. Zakin E, Abrams R, Simpson DM. Diabetic neuropathy. Semin Neurol. 2019;39(5):560-569.
12. Gupta M, Knezevic NN, Abd-Elsayed A, et al. Treatment of painful diabetic neuropathy—a narrative review of pharmacological and interventional approaches. Biomedicines. 2021;9(5):573.
13. Demarin V, Basić-Kes V, Zavoreo I, et al. Recommendations for neuropathic pain treatment. Acta Clin Croat. 2008;47(3):181-191.
14. Bates D, Schultheis BC, Hanes MC, et al. A comprehensive algorithm for management of neuropathic pain. Pain Med. 2019;20(suppl 1):S2-S12.
15. Bernetti A, Agostini F, de Sire A, et al. Neuropathic pain and rehabilitation: a systematic review of international guidelines. Diagnostics (Basel). 2021;11(1):74.
16. Dobson JL, McMillan J, Li L. Benefits of exercise intervention in reducing neuropathic pain. Front Cell Neurosci. 2014;8:102.
17. Song CH, Petrofsky JS, Lee SW, et al. Effects of an exercise program on balance and trunk proprioception in older adults with diabetic neuropathies. Diabetes Technol Ther. 2011;13(8):803-811.
18. Lidoderm (lidocaine patch 5%) product information. Malvern, PA: Endo Pharmaceuticals Inc; January 2015.
19. Lyrica (pregabalin) product information. New York, NY: Pfizer Inc; June 2020.
20. Qutenza (capsaicin patch) product information. Morristown, NJ: Averitas Pharma, Inc; May 2020.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





