US Pharm. 2015;40(8):35-38.
ABSTRACT: Several types of urinary incontinence (UI) are described in the literature: functional, overflow, stress, urge, and mixed. While UI is less prevalent in men than in women, older adults of both sexes often experience lower urinary tract symptoms associated with overflow incontinence. Without adequate treatment, patients can develop a negative self-perception and experience poor quality of life. Iatrogenic UI is typically reversible and implicates drug classes that impair urine flow, bladder contractions, or cognition. Nonpharmacologic therapies are attempted for mild UI, whereas moderate-to-severe symptoms require medications and/or surgery, depending on the predominating symptomology. Regular patient follow-up is imperative for assessing the safety and efficacy of interventions for each type of UI.
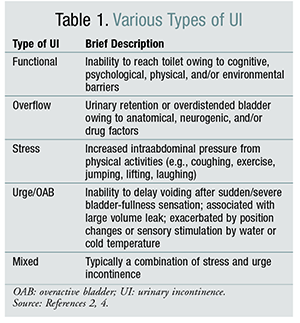
Urinary incontinence (UI) is an inability to control the physical, physiological, or functional factors involved in the process of urination that leads to an involuntary loss of urine.1,2 UI can be transient, acute, or reversible, or it can be established or chronic. UI is not a normal consequence of aging, but it is often reported in older adults.1-3 There are several types of UI: functional, overflow, stress, urge, and mixed (TABLE 1).2,4
Patients with UI eventually develop poor self-rated health (or negative self-perception) and quality of life.1,5 Symptoms of UI can interfere considerably with daily activities and interpersonal relationships, causing patients to experience social isolation, psychiatric conditions (e.g., anxiety, depression, suicidal ideation), other comorbidities (e.g., falls, fractures), and/or financial burdens.1,2,5
Epidemiology
It is difficult to determine accurate prevalence data for UI because of varying definitions and methodologies in the literature.6 In the United States, at least 20 million women and 6 million men experience UI during their lifetime.2 Although UI is much more prevalent in women, the prevalence between the genders is similar after 80 years of age.2,5 At least one-third of patients in community settings and at least one-half of those in long-term care facilities appear to have a form of UI.5,6 Elderly patients with UI also have a significantly increased risk of admission to a nursing facility.5
Etiology
While women most often experience urge incontinence (from bladder irritation or loss of neurologic control) or stress incontinence (from childbirth or obesity), men with prostate problems (e.g., benign prostatic enlargement, hyperplasia, obstruction, or prostatitis) can develop overflow incontinence.2,7 Treatment for prostate disorders (e.g., prostatectomy) may subsequently lead to stress incontinence.2,7
In addition to prostate disorders, men may experience other lower urinary tract symptoms (LUTS). These symptoms may be due to a distal ureteral stone, bladder tumor, urethral stricture, foreign body, urinary tract infection (UTI), nocturnal polyuria, neurogenic bladder dysfunction, or detrusor muscle underactivity or overactivity.7 TABLE 1 discusses some causes of the different types of UI.2,4
Diagnosis
There are various guidelines for each type of UI. Generally, the first step in the diagnostic evaluation of a patient presenting with UI is to determine whether the symptoms are transient/reversible or chronic.2,8 The DIAPPERS mnemonic and the patient’s medications should be reviewed in the differential diagnosis of transient/reversible UI.2,5,8 The DIAPPERS mnemonic includes the following risk factors for UI: Delirium/dementia; Infections (e.g., UTI); Atrophic vaginitis/urethritis or atonic bladder; Pharmacologic agents (e.g., diuretics); Psychological disorders (e.g., depression); Endocrine or excessive urine output (e.g., from excess fluid intake, volume overload, hyperglycemia, diabetes insipidus); Reduced/restricted mobility (i.e., functional incontinence) or reversible urinary retention (e.g., from anticholinergics); and Stool impaction.2,5,8 Pharmacists can help providers identify possible drugs or substances that worsen each type of UI (FIGURE 1 and TABLE 2).2,5,9,10
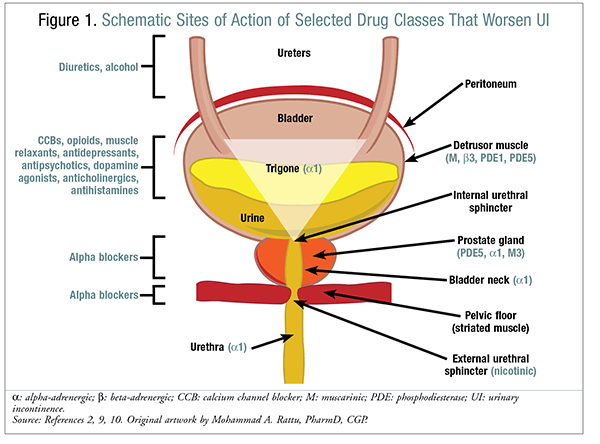
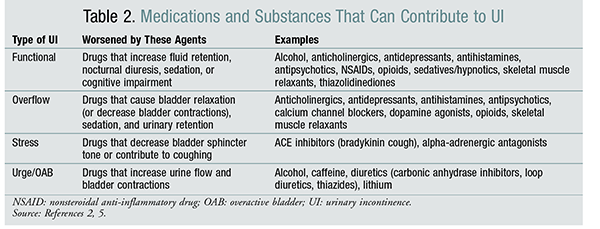
If the patient responds to treatment for the aforementioned reversible causes (e.g., antibiotic for UTI, discontinuation of drugs that induce UI, enhancement of cognition or physical ability to void appropriately), no further intervention is necessary.2 If not, the patient should be assessed for chronic incontinence. Assessment includes obtaining a thorough medical history, administering a symptom questionnaire (e.g., Brown et al’s “3 Incontinence Questions” survey11 to determine urge or stress incontinence predominance), reviewing a voiding diary, performing a physical examination (including a cough stress test), measuring postvoid residual (PVR) urine via ultrasound, and obtaining a laboratory evaluation (e.g., urinalysis).2,8,11 Of course, if the patient has any alarm symptoms (pain, hematuria, proteinuria, abnormal digital rectal examination or marked prostate enlargement, recurrent UTI, prior pelvic surgery/radiotherapy, fistula, PVR >200 mL), a referral to a specialist is warranted.2,8
Current Management
The specific management of UI depends on the patient’s predominant symptoms. For overflow incontinence, if the patient’s symptoms are mild, watchful waiting and lifestyle modifications are appropriate.7 Patients may need to void on a regular schedule (e.g., every 2 hours) to minimize bladder volume and distention.7,12 For moderate-to-severe symptoms, patients should receive interventions that promote acetylcholine transmission to the bladder (e.g., discontinue anticholinergic medications prior to a trial of bethanecol) and/or decrease bladder outlet obstruction (caused by prostate enlargement, urolithiasis, inflammation, or infection).7,12,13
Medications for benign prostatic hyperplasia (BPH) include alpha1-adrenergic antagonists (e.g., alfuzosin, doxazosin, silodosin, tamsulosin, terazosin), 5-alpha-reductase inhibitors (e.g., dutasteride, finasteride), and phosphodiesterase 5 (PDE5) inhibitors (e.g., tadalafil).7 Alpha1-adrenergic antagonists provide the most immediate relief for bladder outlet obstruction caused by BPH, but they increase the risk of orthostatic hypotension; 5-alpha-reductase inhibitors predominantly reduce prostate size and, subsequently, prostate-specific antigen levels.7 Alpha1-adrenergic antagonists and 5-alpha-reductase inhibitors can be combined for the treatment of moderate-to-severe symptoms.7 PDE5 inhibitors can also be used safely with 5-alpha-reductase inhibitors, but they should not be routinely combined with alpha1 receptor antagonists, owing to the potential for additive hypotensive side effects.7
Antimuscarinic therapy may be prescribed in patients with moderate-to-severe bladder-storage symptoms (although cautiously, as it may eventually worsen bladder outlet obstruction).7 Surgical treatment (e.g., bipolar or monopolar transurethral resection of the prostate, prostatectomy, intraprostatic onabotulinumtoxinA) may be warranted for symptoms that are not resolved by pharmacologic therapy.7
For stress incontinence, which may be a result of prostate surgery, patients should receive information on pelvic-floor exercises, as well as on minimizing triggers (climbing stairs, coughing, exercising, jumping, laughing, lifting, pulling, sneezing).8 Patients should also be offered medications that increase contraction and sphincter muscle tone (e.g., pseudoephedrine, duloxetine, imipramine).8,14,15 If these pharmacologic interventions do not provide any benefit, some surgical options are available (collagen injection, artificial urinary sphincter).16
Therapies for urge incontinence, or overactive bladder, are widely practiced. Behavioral therapy (e.g., bladder training, bladder control strategies, pelvic-floor muscle training, fluid management) is a first-line treatment.4,8 Although losing weight, reducing caffeine ingestion, and limiting fluid intake may not result in complete symptom relief, these nonpharmacologic interventions can be as effective as medications for significantly reducing incontinence, improving urinary frequency and nocturia, and enhancing quality of life in some patients.4,8 Second-line treatment includes the use of antimuscarinic medications (darifenacin, fesoterodine, mirabegron, oxybutynin, solifenacin, tolterodine, trospium). These agents have similar efficacy but differing pharmacokinetics (and therefore slightly different adverse-effect profiles), as highlighted in TABLE 3.4,8,17-30 Tricyclic antidepressants (desipramine, doxepin, imipramine, nortriptyline) are additional options, but they are not specific for muscarinic receptors in the bladder.17
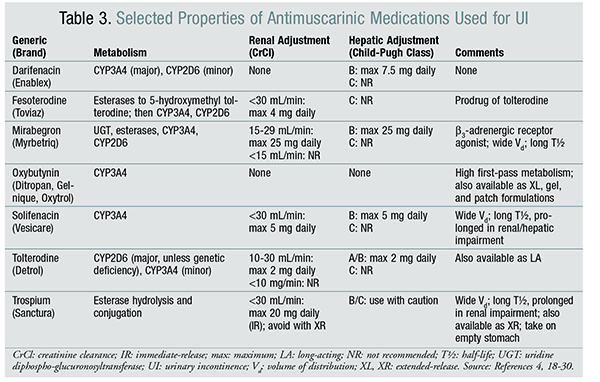
The benefits of antimuscarinic medications should be reviewed within 1 month of initiation.8 Of note, more than 50% of patients may discontinue antimuscarinic medications within the first 3 months because of lack of benefit, adverse effects, or cost of therapy.8 Extended-release, topical, and transdermal formulations generally confer lower rates of xerostomia.4,8 Patients who experience inadequate symptom control and/or unacceptable adverse effects should have their antimuscarinic dose modified or receive a different antimuscarinic.4,8 The mild side effects of occasional constipation or xerostomia can initially be controlled with bowel or fluid management, respectively.4 Antimuscarinic medications should not be prescribed for patients with narrow-angle glaucoma, impaired gastric emptying, or a history of urinary retention.4,8 Caution should always be exercised when prescribing antimuscarinic medications for patients who are already taking anticholinergics or who may be more susceptible to adverse effects (e.g., frail and older adults).4,8
Patients who are refractory to first- and second-line therapies require referral to a specialist.4 Third-line treatments include neuromodulation (e.g., sacral nerve stimulation, percutaneous tibial nerve stimulation) and intradetrusor onabotulinumtoxinA.4 Indwelling catheters are not recommended because of the adverse risk/benefit balance, unless it is a last-resort option in selected patients.4 Rare cases may require augmentation cystoplasty or urinary diversion.4 Follow-up appointments are imperative for assessing adherence, safety, efficacy, and alternative treatment options.4
For iatrogenic functional incontinence, the suspected medications and/or their respective doses should be modified.31,32 Otherwise, patients should have scheduled or prompted toileting and removal of physical barriers.31,32 If functional incontinence is not remedied, patients may soil themselves continuously, leading to hygienic and dermatologic complications (e.g., skin breakdown, ulceration, infections).31,32
A trial of desmopressin may be warranted in patients who experience nocturnal diuresis caused by any of the aforementioned UI types.7,8
Patient Education
As part of the nonpharmacologic interventions mentioned previously, patients should be counseled on bowel habits, medications (appropriate use, side effects), comorbidities, fluid intake, weight loss, scheduled voiding (if cognitively impaired), and reduced caffeine intake.8 Pads should also be offered, if deemed necessary.8 Patients should be encouraged to speak openly with their providers about LUTS in order to avoid developing a negative self-perception and poor quality of life, which can lead to additional comorbidities.1,2,5
Conclusion
UI, which occurs in both genders—although at a lower prevalence in men—is a cluster of conditions that can significantly impair a patient’s well-being. Accurate diagnosis of each type of UI may appear complicated in patients who present with a range of symptoms, but medical management focuses on alleviating the predominating symptomology. First-line therapies for UI are generally nonpharmacologic, whereas medications and surgery are reserved for patients who develop moderate-to-severe symptoms. Since drug-induced UI is often part of the differential diagnosis, it is crucial for pharmacists to be involved in reviewing, suggesting modifications to, and monitoring patients’ pharmacotherapy, alongside other members of the healthcare team.
REFERENCES
1. FDA. Guidance for industry and FDA staff—clinical investigations of devices indicated for the treatment of urinary incontinence. www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm070852.htm. Accessed May 17, 2015.
2. Khandelwal C, Kistler C. Diagnosis of urinary incontinence. Am Fam Physician. 2013;87:543-550.
3. Urinary incontinence. In: Litwin MS, Saigal CS, eds. Urologic Diseases in America. U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Washington, DC: U.S. Government Printing Office; 2012:97-160. NIH Publication No. 12-7865.
4. Gormley EA, Lightner DJ, Burgio KL, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. J Urol. 2012;188(suppl 6):2455-2463.
5. Erdem N, Chu FM. Management of overactive bladder and urge urinary incontinence in the elderly patient. Am J Med. 2006;119(3 suppl 1):29-36.
6. Buckley BS, Lapitan MC; Epidemiology Committee of the Fourth International Consultation on Incontinence, Paris, 2008. Prevalence of urinary incontinence in men, women, and children—current evidence: findings of the Fourth International Consultation on Incontinence. Urology. 2010;76:265-270.
7. Oelke M, Bachmann A, Descazeaud A, et al. EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol. 2013;64:118-140.
8. Lucas MG, Bosch RJ, Burkhard FC, et al. EAU guidelines on assessment and nonsurgical management of urinary incontinence. Eur Urol. 2012;62:1130-1142.
9. Anticholinergic therapy for OAB: cognitive implications for the elderly [transcript of Web conference]. www.medscape.org/viewarticle/542968. Accessed May 17, 2015.
10. Rahnama’i MS, Ückert S, Hohnen R, van Koeveringe GA. The role of phosphodiesterases in bladder pathophysiology. Nat Rev Urol. 2013;10:414-424.
11. Brown JS, Bradley CS, Subak LL, et al. The sensitivity and specificity of a simple test to distinguish between urge and stress urinary incontinence. Ann Intern Med. 2006;144:715-723.
12. Bing MT, Uhlman MA, Kreder KJ. An update in the treatment of male urinary incontinence. Curr Opin Urol. 2013;23:540-544.
13. Taylor JA III, Kuchel GA. Detrusor underactivity: clinical features and pathogenesis of an underdiagnosed geriatric condition. J Am Geriatr Soc. 2006;54:1920-1932.
14. Guay DR. Duloxetine for management of stress urinary incontinence. Am J Geriatr Pharmacother. 2005;3:25-38.
15. Tsakiris P, de la Rosette JJ, Michel MC, Oelke M. Pharmacologic treatment of male stress urinary incontinence: systematic review of the literature and levels of evidence. Eur Urol. 2008;53:53-59.
16. McGuire EJ, English SF. Periurethral collagen injection for male and female sphincteric incontinence: indications, techniques, and result. World J Urol. 1997;15:306-309.
17. Sullivan J, Abrams P. Pharmacological management of incontinence. Eur Urol. 1999;36(suppl 1):89-95.
18. Adigun M, Adesoye A, Ayoola A, et al. Review of nonneurogenic overactive bladder. www.uspharmacist.com/continuing_education/ceviewtest/lessonid/110329. Accessed May 17, 2015.
19. Enablex (darifenacin) product information. Rockaway, NJ: Warner Chilcott (US), LLC; October 2013.
20. Toviaz (fesoterodine fumarate) product information. New York, NY: Pfizer Labs; January 2014.
21. Myrbetriq (mirabegron) product information. Northbrook, IL: Astellas Pharma US, Inc; February 2014.
22. Ditropan (oxybutynin chloride oral tablets) product information. Raritan, NJ: Ortho Women’s Health & Urology; December 2011.
23. Ditropan XL (oxybutynin chloride) product information. Titusville, NJ: Janssen Pharmaceuticals, Inc; February 2015.
24. Oxytrol (oxybutynin) product information. Parsippany, NJ: Watson Pharma, Inc; October 2012.
25. Gelnique (oxybutynin) product information. Parsippany, NJ: Watson Pharma, Inc; January 2013.
26. Vesicare (solifenacin succinate) product information. Northbrook, IL: Astellas Pharma US, Inc; October 2013.
27. Detrol (tolterodine tartrate) product information. New York, NY: Pharmacia & Upjohn Co; October 2014.
28. Detrol LA (tolterodine tartrate) product information. New York, NY: Pharmacia & Upjohn Co; September 2013.
29. Sanctura (trospium chloride) product information. Irvine, CA: Allergan, Inc; July 2012.
30. Sanctura XR (trospium chloride) product information. Irvine, CA: Allergan, Inc; August 2012.
31. Sakakibara R, Uchiyama T, Yamanishi T, Kishi M. Dementia and lower urinary dysfunction: with a reference to anticholinergic use in elderly population. Int J Urol. 2008;15:778-788.
32. Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55:780-791.
To comment on this article, contact rdavidson@uspharmacist.com.





