US Pharm. 2015;40(11):HS24-HS30.
ABSTRACT: Panic disorder (PD) has an estimated prevalence of approximately 5% in the United States. Though the etiology and pathophysiology of PD are not completely understood, there are several proposed mechanisms thought to contribute to its development. PD can manifest as a sudden onset of fear followed by somatic symptoms such as chest pain, tachycardia, and dizziness. Currently, several treatment modalities are available for the management of PD. In choosing the most effective pharmacotherapeutic regimen, practitioners should carefully consider the severity of symptoms, patient response, and comorbid conditions.
Panic disorder (PD) is a chronic mental disorder with essential features such as recurrent panic attacks, persisting concern about the attacks, and a change in behavior as a result of the attacks.1 The lifetime prevalence of PD in the United States is estimated to be 4.7%, and the condition is two times more likely to occur in women than in men.2,3 When stratified by gender, the lifetime prevalence in women is 5% compared to 2% in men.4 The age of onset for PD is a wide range between 25 and 53 years regardless of gender.5 Patients with PD have a higher incidence of suicidality, impaired social functioning, and substance abuse.6-9
Etiology
The etiology of PD is not fully under-stood; however, it is thought that genetics, neurobiology, stress, and life events play significant roles in its development. Several studies have shown that the risk of PD is eight times higher in those with first-degree relatives with PD compared to those with no family history.10 Likewise, childhood events such as physical or sexual abuse, asthma, and smoking have all been associated with an increased risk of PD in adulthood.11-14
Pathophysiology
Though the exact pathophysiology of PD is currently unknown, it is thought that abnormal functioning of gamma-aminobutyric acid (GABA), norepinephrine (NE), serotonin (5-HT), and corticotropin-releasing factor (CRF) neurotransmitter systems play a role.15 GABA is an inhibitory central nervous system (CNS) neuro-transmitter that reduces neuronal excitability when it binds to the GABAA receptor. The GABA receptor model postulates that PD is a result of a lack of central inhibition and decreased GABA concentrations, leading to uncontrolled anxiety during panic attacks.15 The noradrenergic theory states that patients experiencing PD have presynaptic NE autoreceptors that are hypersensitive to stimulation by NE.15 Research surrounding the noradrenergic model has revealed that administration of drugs that stimulate noradrenergic activity, thus releasing NE and increasing glutamate levels that result in feelings of anxiety, precipitate panic attack in those with PD and not in normal volunteers.15
Currently, two research models are being conducted around the role of 5-HT in panic disorders. First, the 5-HT excess model suggests that PD patients have an increased level of 5-HT or that they are hypersensitive to 5-HT. In contrast, the 5-HT deficit model proposes that there are regions in the brain where 5-HT reduces panic behavior and that a deficit of 5-HT would, therefore, facilitate a panic response.16 Consequently, clinical studies have shown that drugs used to increase 5-HT levels by blocking the reuptake are effective in the treatment of PD.16

Clinical Manifestations
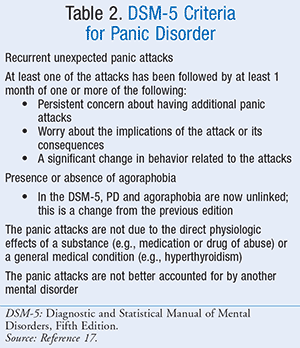
Patients who experience acute panic attacks may present with a variety of symptoms. To date, the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) has outlined a set of 13 symptoms typically associated with acute panic attacks (TABLE 1).17 To diagnose, patients must present with at least four symptoms of PD peaking approximately 10 minutes after onset in addition to a sudden onset of fear or discomfort.17 These symptoms are often grouped into four subtypes, which include cardiac, respiratory, gastrointestinal (GI), and vestibular.18 However, to be diagnosed with PD, patients must meet the criteria set forth by the DSM-5 (TABLE 2).17
Differential Diagnosis
Regardless of the subtype, panic attacks are acute, intense, and transient in nature with no laboratory values or chronic symptomatology associated with, or that can be used in, its diagnosis.18 Therefore, a differential diagnosis should be conducted to rule out disorders that have similar clinical presentation. Cardiac subtype attacks are often confused with myocardial infarction, while GI subtypes can experience nausea, dyspepsia, and symptoms that overlap with signs of irritable bowel disease (IBD). Lastly, vestibular panic attacks present with dizziness, lightheadedness, and unsteadiness, which can often be mistaken for other vestibular disorders such as vertigo.18
Nonpharmacologic Management
Nonpharmacologic treatment options for panic attack include cognitive behavioral therapy (CBT), patient support groups, and panic-focused psychodynamic psychotherapy.1 CBT focuses on confronting stimuli and situations that have caused panic attacks in the past. It consists of weekly sessions for 3 to 4 months where the patient identifies cues that may precede panic attacks and learns techniques to control symptoms.1 Group therapy sessions have been shown to produce benefits, especially with reducing the stigma and shame patients may feel.1 Psychodynamic psychotherapy uses the exploration of past feelings and stressors to achieve remission of panic disorders.1 These programs would be beneficial in patients who are pregnant or want to avoid medications.
Acute Management
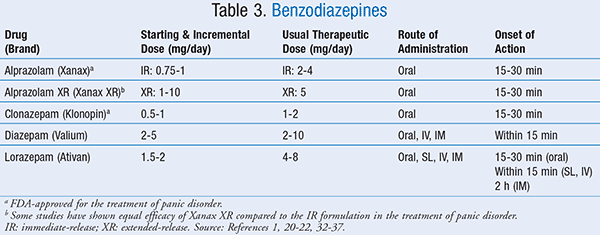
Acute management of PD typically involves sedative measures in efforts to relax the patient and reduce his or her anxiety. As previously mentioned, the neurotransmitter GABA has been shown to reduce acute episodes of panic. Benzodiazepines (BZDs) increase the efficiency of the GABAA receptor via allosteric binding, resulting in reduced anxiety and sedation.19 BZDs have a rapid onset of action and are also more efficacious against panic and anticipatory anxiety when compared to selective serotonin reuptake inhibitors (SSRIs).20 It is important to note that BZDs can cause sedation and retrograde amnesia. Other adverse effects include fatigue, ataxia, slurred speech, memory impairment, weakness, and possibility of abuse and addiction. Additionally, BZDs have a high potential for withdrawal symptoms.18 Although withdrawal symptoms may not occur when a BZD is used for the acute management of PD, they should be considered if the BZD is going to be used for long-term therapy (TABLE 3).1,20-22
Chronic Management
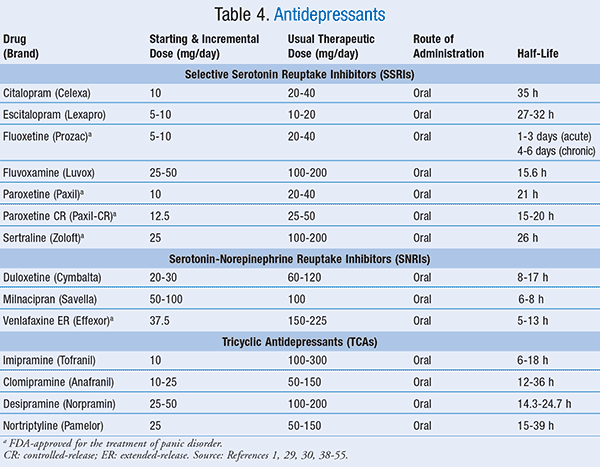
Four primary classes of medications are used in the long-term management of PD. These agents include BZDs and several types of antidepressants, including SSRIs, serotonin-norepinephrine reuptake inhibitors (SNRIs), and tricyclic antidepressants (TCAs) (TABLE 4).1 No specific drug is regarded as the drug of choice; however, SSRIs and SNRIs are recommended as first-line agents according to the American Psychiatric Association (APA) treatment guidelines.2 It is important to note that these drugs typically take 4 to 6 weeks before demonstration of improvement, so BZDs are often added in cases where rapid control of symptoms is desired. Additionally, when treating patients who have comorbid depression or display risks of drug abuse, it may be beneficial to use SNRIs, SSRIs, or TCAs. For antidepressant treatment, low initial doses and slow titration may be of benefit due to sensitivity to side effects in this disorder.1
After therapy is initiated, patients should be monitored approximately every other week until the dose can be optimized and follow-up visits may be decreased. Guidelines suggest treatment duration of 1 year or more to promote symptom reduction and prevent recurrence.1 Moreover, healthcare providers should consider the patient’s history of response, stability, relapse, and current or impending stressors before discontinuation of therapy.1
SSRIs: There are currently six agents available within this class: citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine (immediate- and controlled-release), and sertraline. The functionality of these agents in the management of PD has shown that tryptophan (a serotonin precursor) may exacerbate panic attacks. Thus, SSRIs play a role in increasing serotonin levels, preventing recurrent panic events.23
Though all four classes of medication have displayed approximately equivalent efficacy in studies, SSRIs are more commonly prescribed for long-term treatment due to their side-effect profile and reduced risk of overdose.24 Selecting between agents is primarily based on each individual agent’s adverse-event and pharmacokinetic profile. For instance, citalopram has displayed reduced insomnia compared to other agents, while sexual dysfunction is most commonly associated with paroxetine. Of the SSRIs, fluoxetine has the longest half-life (4 to 6 days in chronic administration) but has increased interactions with other drugs due to CYP2C9, 2C19, 2D6, and 3A4 inhibition.25 Additionally, with abrupt discontinuation, SSRIs can cause neurosensory, neuromotor, and GI problems; however, fluoxetine is less likely to be associated with these issues because of its long half-life.1 Common adverse effects of SSRIs include headache, irritability, nausea, insomnia, sexual dysfunction, weight gain, increased anxiety, drowsiness, and tremor.1
SNRIs: A commonly utilized agent within this class, venlafaxine, has been associated with dose-related hypertension, so it is recommended to measure baseline blood pressure prior to treatment and monitor during therapy.1 Duloxetine and milnacipran have been used in a number of case reports; however, minimal studies have displayed the benefit of their use in PD.26-29 The mechanism for these agents is similar to that of SSRIs in preventing the serotonin depletion that can lead to panic attacks.30 As with SSRIs, discontinuation syndrome may occur in this class as well; thus, dose tapering should be facilitated to minimize risk. Common adverse effects of this class include nausea, dry mouth, constipation, anorexia, insomnia, sweating, somnolence, tremor, and sexual dysfunction.1
TCAs: TCAs have displayed reduced tolerability and safety in comparison to SSRIs, which has contributed to their decreased use in PD.23 Their antagonistic effects on a variety of receptors (muscarinic, alpha1 adrenergic, and histamine) induces a range of adverse responses, including anticholinergic effects, orthostatic hypotension, weight gain, and cardiovascular effects.1 Other adverse effects include increased sweating, sleep disturbances, dizziness, fatigue, weakness, cognitive disturbance, and sexual dysfunction.1 Although a variety of TCA agents have displayed positive effects in controlled trials, imipramine and clomipramine have been the most commonly studied.1 All of the aforementioned agents are much more selective for preventing reuptake of serotonin compared to norepinephrine, providing a basis for their place in treating PD.31
BZDs: BZDs are among the most commonly prescribed drugs in the treatment of PD due to their rapid onset of action.23 However, they are generally used in conjunction with antidepressants because SSRIs take approximately 4 to 6 weeks to become effective, or they are added on to therapy after antidepressant trials have failed or are not tolerated.23 BZDs have also been associated with a range of disadvantages, which include less efficacy for comorbid depression, increased dosing frequency, and risk of abuse and dependence.23 It is recommended that these agents be dosed on a regular schedule rather than as needed for the prevention of panic attacks in chronic treatment.1 BZDs should be tapered over 2 to 4 months when discontinuing treatment in order to avoid withdrawal symptoms and symptomatic rebound.1
Other Agents: Several small clinical studies of bupropion have shown mixed efficacy in the management of PD.1 Trazodone, nefazodone, mirtazapine, pregabalin, gabapentin, and second-generation antipsychotics have limited data and are not recommended as first-line treatment.1 Controlled trials for illnesses resembling PD have used low doses of the monoamine oxidase inhibitor (MAOI) phenelzine as a treatment; however, the side-effect profile of MAOIs is a major concern.1 Therefore, they should be reserved for patients who do not respond to several other treatment modalities.1
Conclusion
To date, the exact pathogenesis of PD and guidelines for the treatment of this disorder have not been concretely established. Multiple lines of inquiry and research are being conducted on the mechanism of panic attacks, and as more information is revealed, the targeted treatment of PD will continue to improve. Consequently, there is no single therapy that is right for all patients with PD, and differential diagnosis of a panic attack is critical to providing appropriate pharmacotherapeutic treatment.
REFERENCES
1. McIntyre JS, Stein MB, Goin MK, et al; Work Group on Panic Disorder. Practice Guideline for the Treatment of Patients With Panic Disorder. 2nd ed. Arlington, VA: American Psychiatric Association; 2009. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Accessed September 9, 2015.
2. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617-627.
3. Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8-19.
4. Vollrath M, Angst J. Outcome of panic and depression in a seven-year follow-up: results of the Zurich study. Acta Psychiatr Scand. 1989;80:591-596.
5. Kessler RC, Amminger GP, Aguilar-Gaxiola S, et al. Age of onset of mental disorders: a review of recent literature. Curr Opin Psychiatry. 2007;20(4):359-364.
6. Weissman MM, Klerman GL, Markowitz JS, Ouellette R. Suicidal ideation and suicide attempts in panic disorder and attacks. N Engl J Med. 1989;321:1209-1214.
7. Alonso J, Angermeyer MC, Bernert S, et al. Disability and quality of life impact of mental disorders in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatr Scand Suppl. 2004;420:38-46.
8. Klerman GL, Weissman MM, Ouellette R, et al. Panic attacks in the community: social morbidity and health care utilization. JAMA. 1991;265:742-746.
9. Markowitz JS, Weissman MM, Ouellette R, et al. Quality of life in panic disorder. Arch Gen Psychiatry. 1989;46:984-992.
10. Kessler RC, Davis CG, Kendler KS. Childhood adversity and adult psychiatric disorder in the US National Comorbidity Survey. Psychol Med. 1997;27:1101-1119.
11. Hasler G, Gergen PJ, Kleinbaum DG, et al. Asthma and panic in young adults: a 20-year prospective community study. Am J Respir Crit Care Med. 2005;171: 1224-1230.
12. Craske MG, Poulton R, Tsao JC, Plotkin D. Paths to panic disorder/agoraphobia: an exploratory analysis from age 3 to 21 in an unselected birth cohort. J Am Acad Child Adolesc Psychiatry. 2001;40:556-563.
13. Cosci F, Knuts IJ, Abrams K, et al. Cigarette smoking and panic: a critical review of the literature. J Clin Psychiatry. 2010;71:606-615.
14. Isensee B, Wittchen HU, Stein MB, et al. Smoking increases the risk of panic: findings from a prospective community study. Arch Gen Psychiatry. 2003;60: 692-700.
15. Martin EI, Ressler KJ, Binder E, et al. The neurobiology of anxiety disorders: brain imaging, genetics, and psychoneuroendocrinology. Psychiatr Clin North Am. 2009;32:549-575.
16. Maron E, Shlik J. Serotonin function in panic disorder: important, but why? Neuropsychopharmacology. 2006;31(1):1-11.
17. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Arlington, VA: American Psychiatric Press; 2013.
18. Sansone RA, Sansone LA. Panic disorder subtypes: deceptive somatic impersonators. Psychiatry (Edgmont). 2009;6(8):33-37.
19. Nutt DJ, Malizia AL. New insights into the role of the GABAA-benzodiazepine receptor in psychiatric disorder. Br J Psychiatry. 2001;179:390-396.
20. Susman J, Klee B. The role of high-potency benzodiazepines in the treatment of panic disorder. Prim Care Companion J Clin Psychiatry. 2005;7(1):5-11.
21. Haas L. Handbook of Primary Care Psychology. New York, NY: Oxford University Press; 2004:607.
22. Valium (diazepam) package insert. Nutley, NJ: Roche Laboratories, Inc; January 2008.
23. Nutt DJ, Forshall S, Bell C, et al. Mechanisms of action of selective serotonin reuptake inhibitors in the treatment of psychiatric disorders. Eur Neuropsychopharmacol. 1999;9(suppl 3):S81-S86.
24. Marchesi C. Pharmacological management of panic disorder. Neuropsychiatr Dis Treat. 2008;4(1):93-106.
25. Whirl-Carrillo M, McDonagh EM, Hebert JM, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2012;92(4):414-417.
26. Simon NM, Kaufman RE, Hoge EA, et al. Open-label support for duloxetine for the treatment of panic disorder. CNS Neurosci Ther. 2009;15(1):19-23.
27. Crippa JA, Zuardi AW. Duloxetine in the treatment of panic disorder. Int J Neuropsychopharmacol. 2006;9(5):633-634.
28. Preve M, Nisita C, Bellini M, Dell’osso L. Duloxetine in panic disorder with somatic gastric pain. Neuropsychiatr Dis Treat. 2013;9:1811-1813.
29. Blaya C, Seganfredo AC, Dornelles M, et al. The efficacy of milnacipran in panic disorder: an open trial. Int Clin Psychopharmacol. 2007;22(3):153-158.
30. Cymbalta (duloxetine hydrochloride) package insert. Indianapolis, IN: Eli Lilly and Company; June 2015.
31. Brunton L, Chabner B, Knollman B. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York, NY: McGraw-Hill Professional; 2010.
32. Pecknold J, Luthe L, Munjack D, Alexander P. A double-blind, placebo-controlled, multicenter study with alprazolam and extended-release alprazolam in the treatment of panic disorder. J Clin Psychopharmacol. 1994;14:314-321.
33. Takahashi H, Sugita T, Yoshida K, Higuchi H, Shimizu T. Effect of quetiapine in the treatment of panic attacks in patients with schizophrenia: 3 case reports. J Neuropsychiatry Clin Neurosci. 2004;16(1):113-115.
34. Lader M. Management of panic disorder. Expert Rev Neurother. 2005;5(2):259-266.
35. Schweizer E, Patterson W, Rickels K, et al. Double-blind, placebo-controlled study of a once-a-day, sustained-release preparation of alprazolam for the treatment of panic disorder. Am J Psychiatry. 1993;150:1210-1215.
36. Sheehan DV, Sheehan KH, Raj BA. The speed of onset of alprazolam-XR compared to alprazolam-CT in panic disorder. Psychopharmacol Bull. 2007;40(2):63-81.
37. Ativan (lorazepam) package insert. Lake Forest, IL: Akorn, Inc; 2008.
38. Celexa (citalopram) package insert. St. Louis, MO: Forest Pharmaceuticals, Inc; July 2014.
39. Lexapro (escitalopram) prescribing information. St. Louis, MO: Forest Laboratories Inc; October 2014.
40. Prozac (fluoxetine hydrochloride) package insert. Indianapolis, IN: Eli Lilly and Company; October 2014.
41. Luvox (fluvoxamine) package insert. Baudette, MN: ANI Pharmaceuticals, Inc; July 2014.
42. Paxil (paroxetine HCl) package insert. Research Triangle Park, NC: GlaxoSmithKline; July 2014.
43. Paxil CR (paroxetine hydrochloride) package insert. Research Triangle Park, NC: GlaxoSmithKline; July 2014.
44. Zoloft (sertraline) package insert. New York, NY: Pfizer; August 2014.
45. Savella (milnacipran) package insert. St. Louis, MO: Forest Pharmaceuticals; October 2013.
46. Effexor (venlafaxine) package insert. Philadelphia, PA: Wyeth Pharmaceuticals Inc; December 2012.
47. Tofranil (imipramine) package insert. Hazelwood, MO: Mallinckrodt Inc; May 2014.
48. Anafranil (clomipramine) package insert. Hazelwood, MO: Mallinckrodt Inc; May 2014.
49. Norpramin (desipramine) package insert. Bridgewater, NJ: Sanofi-Aventis US LLC; June 2014.
50. Pamelor (nortriptyline) package insert. Hazelwood, MO: Mallinckrodt Inc; June 2014.
51. Benetello P, Fulrnaut M, Zara G, et al. Imipramine pharmacokinetics in depressed geriatric patients. Int J Clin Pharmacol Res. 1990;10:191-195.
52. Sutfin TA, DeVane CL, Jusko WJ. The analysis and disposition of imipramine and its active metabolites in man. Psychopharmacology. 1984;82:310-317.
53. Alexanderson B. Pharmacokinetics of desmethylimipramine and nortriptyline in man after single and multiple PO doses—a crossover study. Eur J Clin Pharmacol. 1972;5:1-10.
54. Bennett WM, Aronoff GR, Golper TA, et al. Drug Prescribing in Renal Failure. 3rd ed. Philadelphia, PA: American College of Physicians; 1994.
55. Overo KF, Gram LF, Hansen V. Interaction of perphenazine with the kinetics of nortriptyline. Acta Pharmacol Toxicol. 1977;40:97-105.
To comment on this article, contact rdavidson@uspharmacist.com.






