US Pharm. 2018;43(8)HS-7-HS-12.
ABSTRACT: Prostate cancer screening remains controversial, with most professional organizations endorsing shared decision-making and considerations of life expectancy as a general approach. Advances in risk stratification provide more precise prognosis and individualized treatment approaches. Active surveillance, surgery, and radiation remain the main treatment modalities for localized disease. For newly diagnosed metastatic disease, androgen-deprivation therapy is the standard of care. Chemotherapy as part of initial treatment appears to extend survival for certain risk groups. In the past decade, a number of novel agents have been shown to improve outcomes in men with castration-resistant prostate cancer.
Prostate cancer is the most common noncutaneous malignancy among men in the United States, with 3.3 million existing survivors and an estimated 161,000 new diagnoses in 2017.1,2 Although prostate cancer is often a slow-growing malignancy, it remains the third leading cause of cancer deaths in men.1,2 Most patients are asymptomatic at diagnosis; prior to the availability of prostate-specific antigen (PSA) testing, the most common presenting symptoms were urinary retention, back pain, bone pain, and hematuria.3 Risk factors for prostate cancer include sub-Saharan African ancestry, family history, certain genetic mutations (BRCA 1 or 2), and older age.3,4 Due to the indolent course of the disease and the morbidity associated with overtreatment, prostate cancer screening remains a controversial topic.3 In the past decade, a number of advances have been made in characterizing disease risk and expanding therapeutic options. This review summarizes the principal approaches to prostate cancer screening, diagnosis, and treatment.
SCREENING AND DIAGNOSIS
The primary modality for prostate cancer screening is the evaluation of PSA with or without digital rectal examination (DRE) in asymptomatic men.3,4 PSA is a glycoprotein produced by prostate epithelial cells. In prostate cancer, an increase in PSA levels is caused by increased PSA production as well as disruption of tissue between the prostate and capillaries, thereby allowing for more PSA in the serum.3 However, although PSA is specific to prostate tissue, it is not specific to cancer. Other factors that may increase PSA levels include prostate manipulations (DRE, biopsy), prostatitis, benign prostatic hyperplasia (BPH), and ejaculation.4 Use of 5 alpha-reductase inhibitors leads to about a 50% decrease in PSA levels after 1 year of therapy.4,5 Some experts suggest doubling the PSA results for patients on chronic 5 alpha-reductase inhibitors to correctly interpret them.5
Traditionally, a PSA >4 ng/mL is considered abnormal.4,6 However, PSA values between 4 and 10 ng/mL have poor discriminating ability between BPH and prostate cancer.3,4 Several methods can be used to increase the specificity of PSA results and decrease unnecessary biopsies.4 For men with PSA values between 4 and 10 ng/mL, measurement of the percentage of free PSA should be considered. Free PSA values >25% most likely indicate BPH; these patients do not require a biopsy.7 The rate of change in PSA over time, PSA velocity, is another tool that can be used with a PSA level between 2 ng/mL and 10 ng/mL to improve specificity. Most experts agree that for men with PSA velocity value of more than 0.35 ng/mL per year, a further workup, including biopsy, should be strongly considered.4
Prostate cancer antigen 3 (PCA3) is an FDA-approved test to help determine if repeat biopsy is necessary in men aged more than 50 years who had negative prostate biopsies in the past. In a prospective, multicenter trial, men with a PCA3 score more than 25 were 4.6 times more likely to have a positive biopsy compared with men with scores under 25.4 In the era of PSA-testing, the role of DRE in prostate cancer screening is controversial. Most organizations recommend DRE as a complementary test for men with elevated serum PSA rather than a standalone screening tool.4,8,9 In men with suspected cancer, microscopic evaluation of prostate tissue acquired via needle biopsy remains the gold standard for diagnosis.4
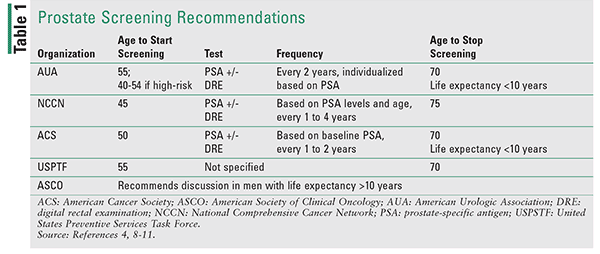
Screening recommendations aim to balance the benefit of prostate cancer detection to prevent mortality versus the harm of overdiagnosis and overtreatment of this often slow-growing disease. Table 1 summarizes screening recommendations from several national medical organizations. While there are some differences, all organizations underscore the importance of shared decision-making that involves patient education regarding the pros and cons of screening and open discussion between the provider and the patient.4,8-11 Screening recommendations are often based on a patient’s life expectancy, with most organizations suggesting avoiding or discontinuing screening in patients with less than 10 years of life expectancy.8,9
TREATMENT
The initial management of newly diagnosed prostate cancer should consider the extended natural history of this malignancy and the risk of progression to more aggressive disease. Shared decision-making, the patient’s life expectancy, and personal preferences play an increasing role in the choice of appropriate treatment options.12 The clinical course of prostate cancer varies from indolent, well-differentiated tumors to aggressive, high-grade cancers that can lead to metastases, significant morbidity, and death. Traditionally, newly diagnosed cases were stratified into low, intermediate, and high risk.12 However, to account for heterogeneity among these groups, a number of more selective risk stratification tools have been developed.12,13 For instance, the National Comprehensive Cancer Network (NCCN) subdivides newly diagnosed localized disease into six risk groups.12 This stratification allows for more individualized treatment approaches, sparing those with low-risk disease from morbidity associated with treatment of localized prostate cancer. The summary of treatment recommendations by risk score is provided in Table 2.
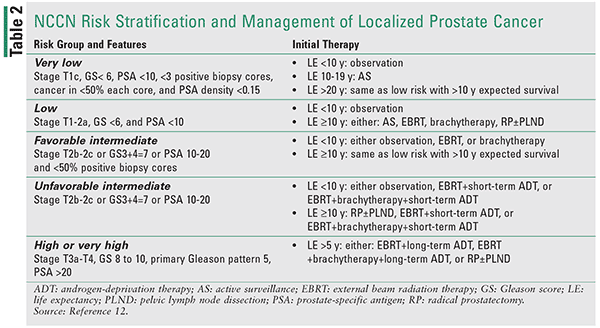
Localized Prostate Cancer
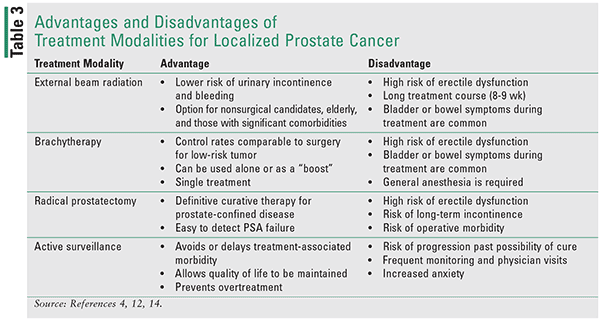
Very Low- and Low-risk Disease: For patients with low-risk disease, the three main treatment modalities are expectant management, radical prostatectomy, and radiation.12 The expectant management is further subdivided into observation and active surveillance.12,14 Observation entails less frequent monitoring and palliative treatment of symptoms, whereas active surveillance consists of more frequent monitoring with a goal of initiating curative treatment if cancer progresses.12,14 The choice of treatment modality takes into consideration the patient’s age, comorbidities, and preferences, with more aggressive treatment options recommended to patients with life expectancy >10 years.12 The pros and cons of these treatment modalities are listed in Table 3.
Intermediate, High Risk, and Locally Advanced Disease: For patients with intermediate-risk disease, concurrent radiation and short-term (4-6 months) androgen deprivation therapy (ADT) may be indicated.12 This recommendation is based on the results of several randomized trials demonstrating significant improvement in overall and cancer-specific survival with the addition of ADT.15-17
For high- and very high-risk patients, a combination of external beam radiation therapy and long-term (2-3 years) ADT is an NCCN category 1 recommendation.12 This combination has shown improvement in overall and cancer-specific survival over either treatment alone.18,19 Supporting evidence for extended duration of ADT comes from the EORTC 22961 trial demonstrating inferior survival with short-term (6 months) versus long-term (2.5 years) ADT among 970 patients with stage T2c-T3 or N1 disease.20 However, ADT should not be used alone or in combination with radical prostatectomy in clinically localized prostate cancer due to lack of survival benefit.12
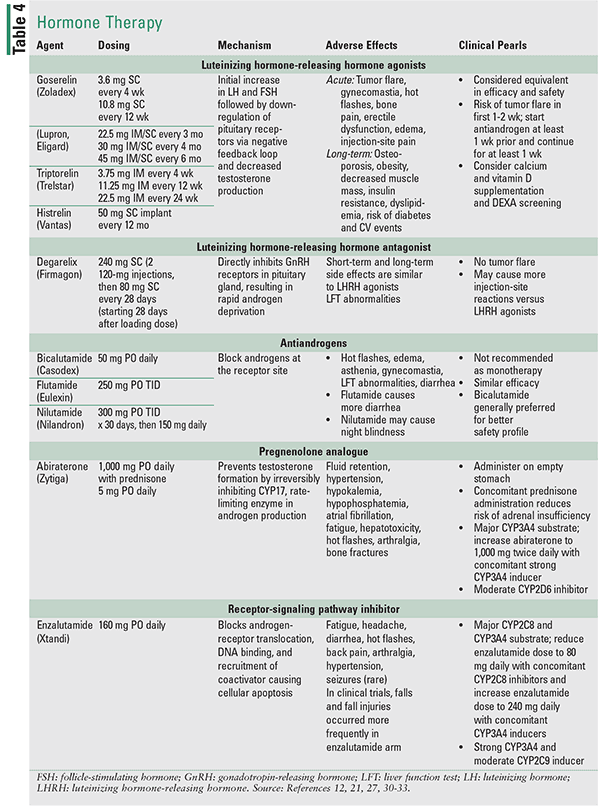
The goal of ADT is to lower testosterone to <50 ng/dL, a level equivalent to castration.12 The therapeutic options include bilateral orchiectomy or medical castration with a luteinizing hormone-releasing hormone (LHRH) agonist or antagonist, which are considered equally effective.12 Monotherapy with an antiandrogen is not currently recommended.12,21 FDA-approved agents are described in Table 4. ADT has a variety of adverse effects, including osteoporosis, dyslipidemia, cardiovascular disease, and increased risk of diabetes and requires thorough patient education and monitoring.
Recurrent and Metastatic Disease
For patients with rising PSA levels, further evaluation is necessary to distinguish between biochemical recurrence and metastatic disease. For patients with biochemical recurrence alone, initiation of ADT or observation could be offered based on PSA velocity, patient’s life expectancy, and comorbidities.12 For patients with shorter PSA doubling time, early consideration of ADT might be preferred.12 Intermittent ADT should be considered in these situations.22 A meta-analysis of six randomized trials of men with locally advanced cancer showed no difference in overall or progression-free survival between intermittent or continuous ADT.22 Patients on intermittent ADT had a better quality of life and fewer side effects. Generally, intermittent ADT refers to discontinuation of therapy once PSA falls below prespecified levels (<4 ng/dL) and reinitiating when PSA rises (usually 10-20 ng/dL).
For men with newly diagnosed metastatic prostate cancer, ADT continues to be the first-line treatment option. Intermittent ADT can be considered in these cases. Several meta-analyses have shown no difference in survival between intermittent and continuous approaches, with significant improvement of quality of life with intermittent ADT demonstrated in most studies.23,24 For men starting on LHRH, combined androgen blockage (CAB) with antiandrogen is recommended to prevent tumor flare. The tumor flare is caused by initial induction of luteinizing hormone and follicle-stimulating hormone by LHRH agonists and usually resolves within 2 weeks of treatment initiation. CAB continued beyond that provides limited-to-no benefit.12
More recent options for newly diagnosed metastatic disease include a combination of ADT with docetaxel or abiraterone with prednisone.12 Two randomized trials assessed the new role of docetaxel, previously reserved for castration-resistant prostate cancer (CRPC). The CHAARTED trial compared ADT alone versus ADT with docetaxel for 6 cycles in 790 ADT-naive metastatic prostate cancer patients.25 Median survival was prolonged from 44.0 to 57.6 months and progression was delayed from 11.7 to 20.2 months with the addition of docetaxel. Greater benefit was seen in high-volume disease, defined as visceral metastases or >4 bone metastases with at least one beyond the pelvis column. The benefit for low-volume disease is less certain and docetaxel therapy is not appropriate for patients without metastases or with low-volume metastatic disease. Additional toxicities associated with docetaxel included mucositis, fatigue, diarrhea, neutropenia, and neuropathy. Results of the STAMPEDE trial confirmed the survival benefit seen in the CHAARTED trial.26 A recent meta-analysis evaluated three trials analyzing the addition of abiraterone with prednisone to ADT.27 The authors found a 38% death-risk reduction with the addition of abiraterone compared with ADT alone, suggesting that this is viable therapeutic option.
Castration-Resistant Prostate Cancer
CRPC refers to disease that progresses while on ADT. Possible mechanisms include an increase in autocrine and paracrine androgen synthesis or androgen-receptor activation.28 ADT is continued beyond progression to maintain testosterone levels equivalent to castration.12,29 Choice of therapeutic options for patients with CRPC depends on the presence or absence of metastases as well as disease symptoms.12
For minimally symptomatic disease, the options are to change antiandrogen, add abiraterone, or switch to second-line hormonal therapy such as ketoconazole, estrogens, or progesterones.12,29 Due to similar mechanism of action, ketoconazole should not be offered to patients who progressed on abiraterone.
In the past decade, several novel treatment options have been shown to improve survival and quality of life for patients with CRPC.30-36 Abiraterone and enzalutamide both work on the androgen axis and are described in more detail in Table 3. Both drugs have been studied in the pre- and postdocetaxel setting and have been shown to improve survival and secondary endpoints, such as pain and quality of life, compared with placebo.30-33 Sipuleucel-T is an alternative treatment option for patients with metastatic CRPC who have minimal symptoms, good performance status (e.g., Eastern Cooperative Oncology Group Scale score 0-1), no liver metastases, and >6 months life expectancy.12 Sipuleucel-T is an autologous cellular immunotherapy that demonstrated a 4.1-month improvement in overall survival (22% reduction in mortality) compared with placebo among patients who met the aforementioned criteria.34 Therapy was generally well tolerated with flulike symptoms as the major adverse effect.34 Another form of immunotherapy, pembrolizumab, may be offered for patients who are microsatellite instability-high or mismatch repair deficient and have progressed through at least one systemic therapy.12
For patients with symptomatic metastatic CRPC, docetaxel with a corticosteroid remains an NCCN category 1 recommendation.12 Only 21-day docetaxel cycles have demonstrated survival advantage.12 For patients who are symptomatic with bone-only metastases Radium-223 may be offered. This alpha-emitting radiopharmaceutical has been shown to improve survival in patients with symptomatic bone metastases as the only site of disease.35 The most common grade 3 to 4 toxicities were hematologic.35 Radium-223 should not be combined with any other chemotherapy due to risk of increased myelosuppression.12 Cabazitaxel is a tubulin-binding taxane derivative that, in combination with a steroid, is considered the standard of care for patients who progress post docetaxel. In the postdocetaxel setting, cabazitaxel combined with prednisone significantly improved overall and progression-free survival compared with mitoxantrone and prednisone.36 However, the incidence of neutropenia, diarrhea, and febrile neutropenia was higher in the cabazitaxel arm.36 Thus, appropriate patient monitoring and use of prophylactic granulocyte growth factors should be considered. A lower cabazitaxel dose of 20 mg/m2 can be considered for frail patients.37 This dose was shown to be noninferior to a standard 25-mg/m2 dose and caused fewer severe adverse events, including neutropenia.37 Currently, there are no therapies that have been shown to offer survival advantage postcabazitaxel progression.12 Mitoxantrone combined with prednisone offers palliation of symptoms, but failed to show improvement in overall survival compared with prednisone alone.12 Men with advanced CRPC should be encouraged to participate in clinical trials.12
CONCLUSION
While some controversy remains regarding prostate cancer screening, most organizations incorporate shared decision-making and patient life expectancy as a core consideration. Pharmacists may play an important role in consulting and educating patients about the screening tests, treatments, and related benefits and harms. Enhanced risk-classification methods and expanded treatment options allow pharmacists to counsel patients regarding therapy based on cancer prognosis and patient preference. ADT remains the standard of care for newly diagnosed metastatic disease and an important option in the management of higher risk localized cancer. Pharmacists are well-equipped to provide patient counseling and management of complications of ADT.
REFERENCES
1. Siegel RL, Miller Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30.
2. Surveillance, Epidemiology, and End Results (SEER) Program. Cancer Statistics (2007-2013), Prostate Cancer. Bethesda, MD: National Cancer Institute.
https://seer.cancer.gov/statfacts/html/prost.html. Accessed February 17, 2018.
3. Eggener SE, Cifu AS, Nabhan C. Prostate cancer screening. JAMA. 2015;314(8):825-826.
4. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. prostate cancer early detection: version 2.2017.
www.nccn.org/professionals/physician_gls/pdf/prostate_detection.pdf. Accessed February 17, 2018.
5. Andriole GL, Marberger M, Roehrborn CG. Clinical usefulness of serum prostate specific antigen for the detection of prostate cancer is preserved in men receiving the dual 5alpha-reductase inhibitor dutasteride. J Urol. 2006;175(5):1657.
6. Gann PH, Hennekens CH, Stampfer MJ. A prospective evaluation of plasma prostate-specific antigen for detection of prostatic cancer. JAMA. 1995;273(4):289-294.
7. Partin AW, Brawer MK, Subong EN, et al. Prospective evaluation of percent free-PSA and complexed-PSA for early detection of prostate cancer. Prostate Cancer Prostatic Dis. 1998;1(4):197-203.
8. Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA guideline. J Urol. 2013;190(2):419-426.
9. Wolf AM, Wender RC, Etzioni RB, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60(2):70.
10. Bibbins-Domingo K, Grossman DC, Curry SJ. The US Preventive Services Task Force 2017 draft recommendation statement on screening for prostate cancer: an invitation to review and comment. JAMA. 2017;317(19):1949-1950.
11. Basch E, Oliver TK, Vickers A, et al. Screening for prostate cancer with prostate-specific antigen testing: American Society of Clinical Oncology Provisional Clinical Opinion. J Clin Oncol. 2012;30(24):3020.
12. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. prostate cancer: version 1.2018. www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed February 17, 2018.
13. Epstein JI, Egevad L, Amin MB, et al. The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016;40(2):244-252.
14. Filson CP, Marks LS, Litwin MS. Expectant management for men with early stage prostate cancer. CA Cancer J Clin. 2015;65(4):265-282.
15. D’Amico AV, Chen MH, Renshaw AA, et al. Androgen suppression and radiation vs. radiation alone for prostate cancer: a randomized trial. JAMA. 2008;299(3):289-295.
16. Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med. 2011;365(2):107-118.
17. Denham JW, Hunt D, McGowan D, et al. Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10-year data from the TROG 96.01 randomised trial. Lancet Oncol. 2011;12(5):451-459.
18. Horwitz EM, Bae K, Hanks GE, et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26(15):2497-2504.
19. Warde P, Mason M, Ding K, et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomized, phase 3 trial. Lancet. 2011;378(9809):2104-2111.
20. Bolla M, Van Tienhoven G, Warde P, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomized study. Lancet Oncol. 2010;11:1066-1073.
21. McLeod DG, Iversen P, See WA, et al. Bicalutamide 150 mg plus standard care vs. standard care alone for early prostate cancer. BJU Int. 2006;97(2):247-254.
22. Dong Z, Wang H, Xu M, et al. Intermittent hormone therapy versus continuous hormone therapy for locally advanced prostate cancer: a meta-analysis. Aging Male. 2015;18(4):233-237.
23. Magnan S, Zarychanski R, Pilote L. Intermittent vs. continuous androgen deprivation therapy for prostate cancer: a systematic review and meta-analysis. JAMA Oncol. 2015;1(9):1261-1269.
24. Botrel TE, Clark O, dos Reis RB, et al. Intermittent versus continuous androgen deprivation for locally advanced, recurrent or metastatic prostate cancer: a systematic review and meta-analysis. BMC Urol. 2014;14:9.
25. Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737-746.
26. James ND, Chen YH, Carducci M, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163-1177.
27. Rydzewska LHM, Burdett S, Vale CL, et al. Adding abiraterone to androgen deprivation therapy in men with metastatic hormone-sensitive prostate cancer: a systematic review and meta-analysis. Eur J Cancer. 2017;84:88-101.
28. Holzbeierlein J, Lal P, LaTulippe E, et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164(1):217-227.
29. Virgo KS, Basch E1, Loblaw DA, et al. Second-line hormonal therapy for men with chemotherapy-naïve, castration-resistant prostate cancer: American Society of Clinical Oncology provisional clinical opinion. J Clin Oncol. 2017;35(17):1952-1964.
30. de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995-2005.
31. Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138-148.
32. Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187-1197.
33. Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424-433.
34. Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411-422.
35. Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213-223.
36. de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147-1154.
37. Eisenberger M, Hardy-Bessard AC, Kim CS, et al. Phase III study comparing a reduced dose of cabazitaxel (20 mg/m2) and the currently approved dose (25 mg/m2) in postdocetaxel patients with metastatic castration-resistant prostate cancer-PROSELICA. J Clin Oncol. 2017;35(28):3198-3206.
To comment on this article, contact rdavidson@uspharmacist.com.