US Pharm. 2009;34(9):58-68.
In the public-health setting, a variety of mental-health conditions ranging from depression to bipolar disorder (BD) are encountered, but these illnesses are not always treated appropriately during pregnancy. One-year prevalence rates for major depressive disorder (MDD) range from 7% to 13%, with peak occurrence between 25 and 44 years of age.1 BD affects between 4% and 6% of women, with onset usually occurring in the teens to early twenties.2 The onset of schizophrenic disorders commonly occurs during the early childbearing years in women.2 It is important for the pharmacist to know which psychotropic agents are considered safe during pregnancy and which ones should be avoided, and to keep other members of the health care team up-to-date. This article reviews the various psychotropic medications and how they should or should not be used during pregnancy.
In the evaluation of drug therapy during pregnancy, teratogenicity and embryotoxicity must be considered. Damage caused by teratogens occurs during the first trimester of pregnancy, and malformations of fetal organs or skeletal structures may be seen.1 Embryotoxicity can occur when drugs are given in the second or third trimester. Manifestations of embryotoxicity in a neonate include jitteriness, crying, and nervousness.1
To make an accurate recommendation to other health care providers (HCPs) regarding which psychotropics are safe during pregnancy, it is important to consider the FDA's pregnancy safety ratings (categories A, B, C, D, and X). Category A means that controlled studies show no fetal risks associated with the drug; for category B drugs, there is no evidence of risk in humans, although risks have been noted in animal studies; for category C drugs, risk cannot be ruled out; category D drugs have positive evidence of risk; and category X drugs are contraindicated for use in pregnancy. No approved psychotropics have a category A rating.
DEPRESSION
Depression affects 1 in 4 women. A woman who has been diagnosed with MDD has a weighty decision to make concerning whether to continue pharmacologic treatment while trying to conceive. Additionally, patients need to consider whether to discontinue treatment until after the first trimester, when organogenesis takes place. Untreated depression in pregnancy can lead to complications for both mother and child.
Several classes of antidepressants are currently available. The most frequently prescribed class of antidepressants comprises the selective serotonin reuptake inhibitors (SSRIs); commonly prescribed SSRIs include citalopram, escitalopram, paroxetine, fluoxetine, and sertraline. Other commonly prescribed classes include tricyclic antidepressants (TCAs) and selective norepinephrine reuptake inhibitors (SNRIs). Pregnancy is often an exclusion criterion in clinical trials of antidepressants; therefore, limited data exist regarding adverse events of these medications in pregnancy. Pregnancy registries created by manufacturers and practitioners' case reports provide information about adverse reactions that have occurred since the medications entered the market.
Pregnancy categories A and B are considered the safest, but none of the antidepressant medications has a category A or B rating. Following is a review of the major antidepressant classes, their pregnancy categories, and published data.
SSRIs
This class constitutes the most common pharmacologic treatment for depression. However, recent studies have found that there may be an increased risk of teratogenicity, especially with paroxetine, when SSRIs are administered in the first trimester. Data from the Slone Epidemiology Center Birth Defects Study and the National Birth Defects Prevention Study indicated a possible increase in risk of congenital cardiac malformations with first-trimester exposure to paroxetine.1 This prompted the manufacturer to change the pregnancy category from C to D.
One study assessed 1,403 women who had received paroxetine, other SSRIs, or other antidepressants during their first trimester.3 There were 101 infants identified as having major congenital malformations, with 24 malformations of cardiac origin. There was a 2-fold increase in the risk of major malformations and a 3-fold increase in the risk of major cardiac malformations when the fetus was exposed to more than 25 mg/day paroxetine. This study was the first of its kind to show a dose-response relationship between fetal exposure to paroxetine in the first trimester and risk of major cardiac malformation; the study should be repeated in order to form more concrete conclusions. Other SSRIs (fluoxetine, sertraline, and citalopram) have not been linked to major malformations when used in the first trimester; however, data are limited.
When given in the third trimester, SSRIs may cause embryotoxicity or poor neonatal adaptation, also known as neonatal toxicity or neonatal behavioral syndrome. Common signs of embryotoxicity include tremor, feeding difficulties, irritability, agitation, rigidity, and respiratory distress. Other, less frequent, signs include seizures, excessive crying, hyperreflexia, and sleep disturbances. These behaviors usually are self-limiting and resolve in 1 to 2 weeks. Neonates exhibiting these behaviors should be carefully monitored.
The mechanism behind this syndrome is poorly understood, but may involve serotonin withdrawal or toxicity. To categorize symptoms, a retrospective study of fetal exposure to antidepressants in the last 3 weeks of pregnancy was conducted in 73 neonates born in secondary and tertiary care hospitals.4 Not all infants had been exposed to antidepressants. Neonates were grouped into three categories based on literature describing serotonergic toxicity, antidepressant discontinuation syndrome, and neonatal immaturity. Three statistically significant clusters of symptoms associated with antidepressant exposure emerged.4 The SSRIs used most were citalopram, paroxetine, sertraline, and fluoxetine. The study's findings were in agreement with other literature positing that poor neonatal adaptation is due to serotonergic toxicity or discontinuation syndrome.
TCAs
These agents have been used to treat depression since the late 1950s; however, owing to the introduction of newer antidepressants with better side-effect profiles, TCAs have since become second-line or even third-line agents. Many TCAs have been linked to anticholinergic side effects (e.g., dry mouth, urinary retention, constipation); because of this, current data support the use of only nortriptyline and desipramine in pregnancy.1
Soon after TCAs became available, studies found an increased risk of malformations when these drugs were given in the first trimester; however, these results could not be reproduced subsequently.2 Additionally, poor neonatal adaptation has been noted in infants whose mothers were given TCAs in the third trimester. Newborns exposed to TCAs in utero have exhibited symptoms, including diarrhea, jitteriness, and muscle weakness, that may be due to rebound cholinergic hyperactivity.1 These potential cholinergic side effects limit the use of TCAs during pregnancy.
SNRIs
SNRIs--a newer class of antidepressants--include venlafaxine and duloxetine, both of which have gained in popularity recently. However, data and case studies concerning these drugs' effects on neonates when used during pregnancy are limited. Based upon available data for venlafaxine, there does not appear to be an increased risk of major malformations in newborns. At this time, no published case reports or retrospective reviews exist for duloxetine.
A retrospective review of studies investigating neonatal signs after serotonin reuptake inhibitor (SRI) exposure in utero found that, similar to SSRIs, late SNRI exposure carries an overall risk ratio of 3.0 (95% CI, 2.0-4.4).5
Other Antidepressants
Antidepressants that have not been categorized include bupropion, mirtazapine, trazodone, and nefazodone. Extensive studies of neonatal outcomes and adverse events when these agents are taken throughout pregnancy have not been conducted. In a prospective study, 104 pregnant women who were given mirtazapine showed no increased risk of major malformations.5 In a study conducted by the Motherisk Program at the Hospital for Sick Children in Toronto, 136 women who received bupropion during their first trimester were compared with 89 women who did not; the bupropion group showed no increase in risk of major malformations compared with controls.5
Treatment Recommendations
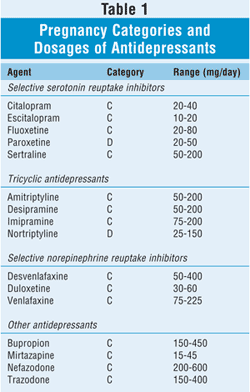
If a patient currently receiving antidepressant therapy wishes to become pregnant, it is important to consider her mental health and medical history as well as her current medications. A decision should be made jointly by the HCP and the patient as to whether discontinuation of the antidepressant is an option. If discontinuing the antidepressant is not desirable, it is essential to examine medical findings regarding its teratogenicity and embryotoxicity to ensure that the least amount of harm is done to the fetus and the mother. Based on current literature, avoidance of paroxetine is recommended in all trimesters.6 Because of limited data on duloxetine, this drug should not be taken during pregnancy. Owing to the increased risk of poor neonatal adaptation, infants with third-trimester exposure to SRIs or TCAs should be monitored. The respective pregnancy categories and dosages of the various antidepressants appear in TABLE 1.
PSYCHOTIC DISORDERS
Schizophrenia, which occurs in approximately 1% to 2% of women, is characterized by psychotic symptoms, negative symptoms, and social dysfunction.2 Women with schizophrenia who become pregnant and remain untreated can manifest behaviors harmful to the neonate, including maternal self-mutilation, refusal of prenatal care, and even infanticide.2 Typical (first-generation) and atypical (second-generation) antipsychotic agents are used to treat schizophrenia.
Typical Antipsychotics
First-generation antipsychotics (FGAs) have been studied extensively since their introduction. Owing to their side-effect profile, they are no longer recommended as first-line agents in the treatment of psychotic disorders. FGAs are subcategorized by structure, as discussed below.
Butyrophenone Derivatives: One of the most studied and utilized FGAs is haloperidol. In most studies, haloperidol has not produced any major malformations.7 In a recent multicenter, prospective, controlled, cohort study, 188 babies exposed to haloperidol in utero had congenital malformation rates within the expected baseline risk for the general population.7 Neonates may experience complications, including withdrawal symptoms, after being exposed to haloperidol late in gestation.
Phenothiazines: The phenothiazines include chlorpromazine, fluphenazine, prochlorperazine, perphenazine, and thioridazine. While most of the data on the teratogenic effects of phenothiazines come from testing in mice and rats, one study examined outcomes of pregnancies in which the mother received phenothiazine.7 Exposure to phenothiazines was found to be brief since these agents are used to treat hyperemesis gravidarum (severe morning sickness), not psychotic disorders. Most of the infants in the study had been exposed to chlorpromazine in utero. There was no significant difference in the occurrence of major fetal malformations in infants with in utero phenothiazine exposure versus those without exposure. However, chlorpromazine has been shown to increase the risk of neonatal respiratory distress syndrome when given in doses exceeding 500 mg/day.7
Atypical Antipsychotics
Second-generation antipsychotics (SGAs) have replaced FGAs in the treatment of psychotic disorders and mood disorders. These include aripiprazole, clozapine, quetiapine, ziprasidone, risperidone, and olanzapine. Although SGAs have become first-line treatment for the aforementioned disorders, data concerning their teratogenicity and other effects in pregnancy remain limited.
Aripiprazole: There are very few case reports concerning fetal exposure to aripiprazole in utero. In these cases, there was no evidence of major malformations. However, symptoms associated with poor neonatal adaptation were evidenced in some of the infants.7
Clozapine: Human reproductive data regarding clozapine have been available since the early 1990s. Case reports and reviews show no association between clozapine exposure and major malformations.8 Fetal overexposure to clozapine can lead to floppy infant syndrome (FIS) and poor neonatal adaptation. Signs of FIS include abnormal posture evidenced by limbs that hang and a head that hangs down. Also, as in adults taking clozapine, a CBC should be performed in neonates exposed to clozapine in utero to monitor for agranulocytosis.8
Olanzapine: Human reproductive data concerning olanzapine are limited and contradictory. Cases of major congenital anomalies and neonatal adverse reactions have been reported, but several other reports describe healthy outcomes in infants who were exposed to olanzapine during all stages of pregnancy.7 Olanzapine also has been implicated in major metabolic complications of pregnancy, such as gestational diabetes. These complications increase the risk of congenital abnormalities.8
Quetiapine: A prospective study compared 36 women treated with quetiapine during early pregnancy and controls who were given nonteratogenic agents during pregnancy.7 Quetiapine was not associated with an increased risk of teratogenicity or undesirable outcomes in mother or baby.7
Risperidone: A review of retrospective and prospective reports of risperidone-exposed pregnancies examined 201 unpublished cases.7 Based on this review, there is no current evidence that risperidone causes an increase in fetal teratogenicity during exposure in any stage of pregnancy.
Ziprasidone: Currently, no case reports exist of ziprasidone administered during pregnancy. However, the drug has been shown to cause teratogenic effects in animals at doses close to human therapeutic doses.7
Treatment Recommendations
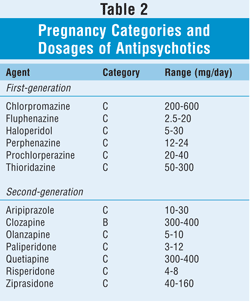
The risks associated with typical antipsychotics are minimal, and these drugs can be used safely during pregnancy. However, to minimize the risk of extrapyramidal side effects that may need to be treated with other medications that are harmful to the fetus, the dose should be restricted to what is necessary to control the patient's symptoms. Owing to limited clinical data, the routine use of SGAs cannot be recommended without restrictions. If the patient's disorder would be uncontrolled without these agents, however, it is important to monitor both mother and fetus very closely. A patient already being treated should not be switched from one antipsychotic class to the other, as this may cause more fetal and maternal complications. TABLE 2 lists the respective pregnancy categories and dosages of the various antipsychotic medications.
MOOD DISORDERS
Typically, mood disorders encompass a spectrum of psychiatric illnesses. BD, the most commonly diagnosed mood disorder, consists of two distinct phases: manic and depressive. People with BD may cycle between the phases or experience just one phase. Pregnant women tend to cycle toward depression, and women who experience an episode during pregnancy are more likely to experience depressive episodes in later pregnancies.9 The two different classes of medications used to treat BD are mood stabilizers and anticonvulsants.
Mood Stabilizers
Lithium has long been the mainstay of BD treatment. Lithium's exact mechanism of action (MOA) is unknown, but the drug is believed to exert its mood-stabilizing effects by interfering with the synthesis, storage, release, and reuptake of monoamine neurotransmitters. Research has found that lithium causes a small increase in congenital cardiac malformations when administered in the first trimester.2 FIS and thyroid toxicity have been evidenced in infants who were exposed to lithium in utero.10
Anticonvulsants
Several medications used to treat seizure disorders are available for the treatment of mood disorders. These anticonvulsants include valproate, carbamazepine, and lamotrigine. The MOA of these anticonvulsants in BD is unknown. Most of the data concerning the effect of these medications on fetal development have involved patients being treated for seizures.
Valproate: This drug has a risk of neural tube defects when given in the first trimester.2,10 This may be due to an increase in free-radical burden associated with the metabolite valproate-4-ene.10 There also is evidence of a fetal valproate syndrome, which features fetal growth restriction, limb and heart defects, and facial changes.
Carbamazepine: Like valproate, carbamazepine has been well studied in women with epilepsy. Exposure to carbamazepine during pregnancy can cause fetal carbamazepine syndrome, which is described as fetal dysmorphisms and fingernail hypoplasia in neonates.8 Carbamazepine exposure causes an increased fetal risk of spina bifida.10
Lamotrigine: Data from the International Lamotrigine Pregnancy Registry suggest that there is no evidence of an increased risk of congenital anomalies when the drug is given during the first trimester.9
Treatment Recommendations
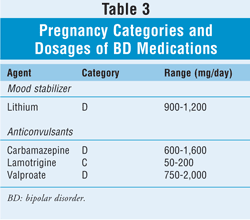
The treatment of BD during pregnancy is generally deemed appropriate, as the risk to the infant from uncontrolled BD is greater than the potential drug effects in utero. If possible, carbamazepine and valproate should be avoided--especially in the first trimester--because of the risk of malformation. Lithium levels should be closely monitored during pregnancy, as gestational changes can affect the drug's absorption, distribution, metabolism, and elimination.2 In patients whose BD episodes are mild or infrequent, lithium should be gradually tapered off before conception; however, patients who experience severe and frequent episodes should continue lithium throughout pregnancy and should be counseled about reproductive risk.2 Lamotrigine is a viable option owing to its favorable side-effect profile and low risk of fetal toxicity; it should be used as monotherapy in patients nonresponsive to lithium.2,9 Lithium alone or in combination with an antipsychotic may be an appropriate alternative to valproate or carbamazepine.9 In patients nonresponsive to lithium, lamotrigine alone or in combination with an antipsychotic may be an adequate option.9 Respective pregnancy categories and dosages of agents used to treat BD appear in TABLE 3.
CONCLUSION
Depression, mood disorders, and other psychiatric disorders are prevalent, with the highest incidence occurring at the onset of the childbearing years in women. It is important to weigh the risks and benefits of all treatment options during pregnancy. Untreated depression, psychosis, and mood disorders can have ill effects on both the patient and the infant; however, pharmacotherapy also may cause negative outcomes for the baby. Because of this, it is important for the patient taking psychotropics to have a thorough discussion with her HCP about whether to continue or discontinue pharmacotherapy during pregnancy.
REFERENCES
1. Feucht C. Treatment of depression during pregnancy. US Pharm. 2007;32(9):34-44.
2. ACOG Practice Bulletin: clinical management guidelines for obstetrician-gynecologists. Obstet Gynecol. 2008;111:1001-1020.
3. Bérard A, Ramos E, Rey E, et al. First trimester exposure to paroxetine and risk of cardiac malformations in infants: the importance of dosage. Birth Defects Res B Dev Reprod Toxicol. 2007;80:18-27.
4. Boucher N, Bairam A, Beaulac-Baillargeon L. A new look at the neonate's clinical presentation after in utero exposure to antidepressants in late pregnancy. J Clin Psychopharmacol. 2008;28:334-339.
5. Way CM. Safety of newer antidepressants in pregnancy. Pharmaco therapy. 2007;27:546-552.
6. Gonsalves L, Schuermeyer I. Treating depression in pregnancy: practical suggestions. Cleve Clin J Med. 2006;73:1098-1104.
7. Gentile S. Antipsychotic therapy during early and late pregnancy. A systematic review. Schizophr Bull. Published online on September 11, 2008.
http://schizophreniabulletin.
8. Gentile S. Clinical utilization of atypical antipsychotics in pregnancy and lactation. Ann Pharmacother. 2004;38:1265-1271.
9. Cohen LS. Treatment of bipolar disorder during pregnancy. J Clin Psychiatry. 2007;68(suppl 9):4-9.
10. Dodd S, Berk M. The safety of medications for the treatment of bipolar disorder during pregnancy and the puerperium. Curr Drug Saf. 2006;1:25-33.






