US Pharm. 2021;46(6):HS-2-HS-1
ABSTRACT: Heart-failure prevalence continues to rise. Despite new treatments, 5-year mortality remains around 50%. Men are more likely to have heart failure with reduced ejection fraction, which has a plethora of treatment options available to decrease morbidity and mortality. New agents to target heart failure have been discovered since the last update to the guidelines in 2017. Health system pharmacists play a significant role in heart failure management, helping to decrease hospital readmission rates via appropriate drug selection and patient counseling.
Heart failure (HF) is prevalent in around 6 million U.S. citizens aged 20 years or older, according to the most recent heart disease statistics that looked at HF prevalence from 2015 to 2018. Of these, 3.4 million were men. The prevalence of HF continues to rise, which emphasizes the importance of prevention and management, as 5-year mortality remains at around 50%. The lifetime risk of HF is 30% to 42% in white males and 20% to 29% in black males. Declining mortality rates have been attributed to evidence-based treatment approaches.1
HFpEF Versus HFrEF
HF is a clinical syndrome that can result from disorders of the heart structure, heart function, or metabolic abnormalities. The symptoms of HF are attributed to impaired left ventricular myocardial function. HF symptoms include dyspnea, fatigue, and fluid retention. Ejection fraction (EF) is a measure of the percentage of blood being pumped per contraction from the left ventricle. It is used to differentiate between the two most common types of HF. It is also important to note because clinical trials use EF as a patient-selection criterion, limiting drug benefit to patients who mimic that trial’s inclusion criteria. Reduced EF is an EF £40%, whereas a preserved EF is ³50%. Because patients can have coexisting systolic and diastolic abnormalities, the terms HF with preserved ejection fraction (HFpEF) and HF with reduced ejection fraction (HFrEF) are preferred.2 When looking at gender differences in HF, men are more likely to have HFrEF.3,4
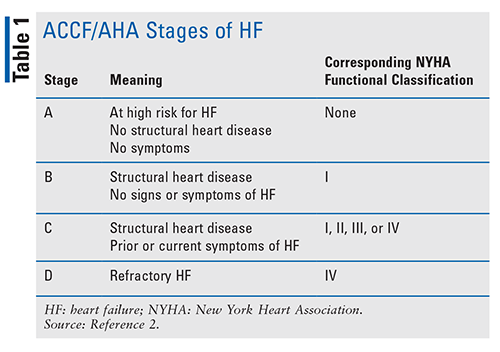
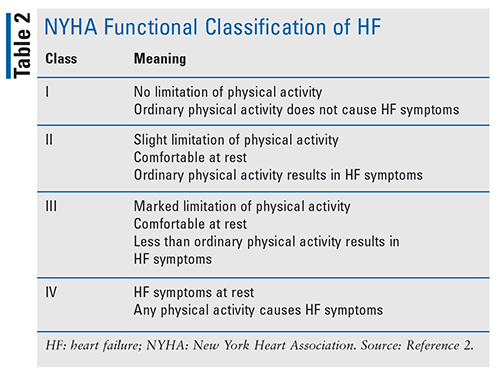
In addition to determining the type of HF, the American College of Cardiology Foundation (ACCF) and American Heart Association (AHA) stages of HF are used to evaluate disease development and progression. The New York Heart Association (NYHA) functional classification is also used to determine exercise capacity and symptom progression (TABLES 1 and 2). It is important to properly classify patients, as different treatments have shown benefit in specific subsets of individuals with HF.2
HF Prevention
As with all chronic disease, it is preferred to prevent the occurrence of the disease when possible. Management of diseases that are known to be causative of HF should be prioritized. Some of the most causative risk factors for HF include hypertension, diabetes mellitus, metabolic syndrome, and atherosclerotic disease.2 One study showed a lower lifetime risk of HF in patients who followed the AHA’s Life’s Simple 7 guidelines.5 This positive association was similar in both women and men.5 The seven principles are as follows: 1) stop smoking; 2) eat better; 3) get active; 4) lose weight; 5) manage blood pressure; 6) control cholesterol; and 7) reduce blood sugar.6
HF Guideline Recommendations
Guideline-based treatment of HF is determined based upon the ACCF/AHA stage of HF, type of HF, and NYHA functional class. Stage A indicates a patient is at high risk for HF (HFrEF and HFpEF), thus management focuses on risk reduction. Stage B HF (HFrEF and HFpEF) lacks symptom presentation and focuses on evidence-based management of underlying causes. An example would be the use of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs), statins, and evidence-based beta-blockers (BBs) in patients with recent myocardial infarction (MI) or acute coronary syndrome (ACS).2
Stage C HF is treated a bit differently, and as such, the classification becomes more important.2 Currently, most of the evidence for morbidity and mortality reduction is in HFrEF. This is good news for the male population, as they are more likely to have HFrEF. All patients with edema in any type of HF will be on diuretics, typically loop, to relieve congestion. Stage C HFpEF patients will be treated with guideline-driven management for their comorbid conditions, as evidence for morbidity and mortality reduction is lacking. In addition to diuretics as needed, patients with stage C HFrEF should be taking an ACEI or ARB and an evidence-based BB. Clinical evaluation, diagnostic testing, and pharmacologic and procedural treatments recommended in the AHA guidelines are referred to as guideline-directed management and therapy (GDMT). GDMT includes ACEIs or ARBs, BBs, and the use of angiotensin receptor-neprilysin inhibitors (ARNIs), aldosterone antagonists, hydralazine/isosorbide dinitrate, ivabradine, or digoxin, depending on other factors.2,7
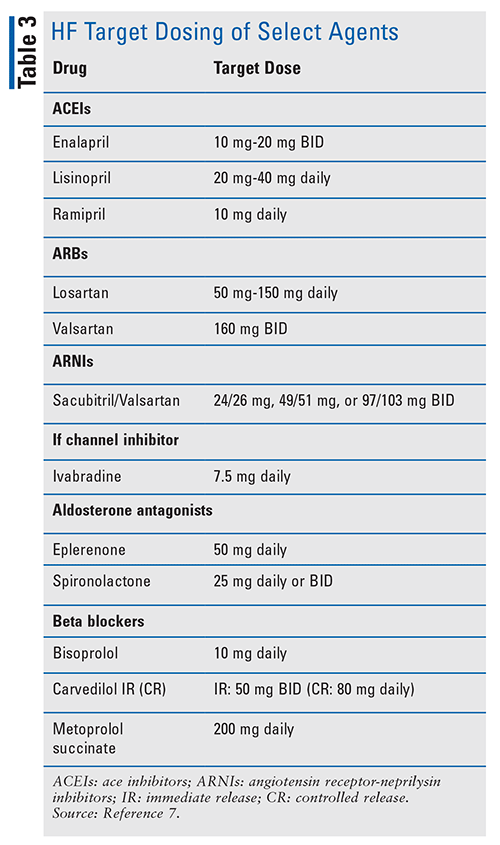
ACEIs and ARBs: It is well established that ACEIs decrease morbidity and mortality in patients with HFrEF, and that those patients who are intolerant of ACEIs due to angioedema or cough should use ARBs as they show similar morbidity and mortality reductions.3,7 Practitioners should strive to attain target HF dosing, as these doses were where morbidity and mortality benefit was seen (TABLE 3). Renal function and blood pressure should be followed in these patients while titrating dosing and during maintenance.3
BBs: When it comes to HF, not all BBs are considered equivalent. There are three BBs that have been shown to decrease morbidity and mortality: metoprolol succinate, carvedilol, and bisoprolol. It is recommended that one of these agents be used in the management of all patients with HFrEF. Patients should be titrated to the target dose or to the maximally tolerated dose. Bradycardia is the most frequent cause of BB intolerance. Patients should be considered intolerant when they are symptomatically bradycardic (dizziness or lightheadedness) instead of relying on a specific pulse rate. BBs can lead to worsening HF by increasing fluid retention. In this case, diuretic doses can typically be adjusted to compensate. Hypotension due to the use of BBs and ACEIs or ARBs concurrently can be combatted by separating administration times.3,7
ARNI: Patients with NYHA Class II through III HF who have an adequate blood pressure on an ACEI or ARB and GDMT BB can benefit from decreased morbidity and mortality by discontinuing the ACEI or ARB and initiating an ARNI. Currently, sacubitril/valsartan (Entresto) is the only available ARNI on the market. In the PARADIGM-HF trial, the sacubitril/valsartan arm had a decreased incidence of death from cardiovascular (CV) causes or hospitalization by 20% compared with the enalapril arm.8 Target doses of sacubitril/valsartan are provided in TABLE 3, and renal function should be monitored. In patients who are being switched from an ACEI to an ARNI, the ACEI should be stopped for 36 hours prior to initiation of the ARNI to decrease the risk of angioedema and associated morbidity.7
Aldosterone Antagonists: Patients with NYHA class II through IV HF with an LVEF £35% should be initiated on an aldosterone antagonist to decrease morbidity and mortality. Elevated plasma natriuretic peptide levels or previous history of hospitalization due to a CV issue are a prerequisite to use of aldosterone antagonists in patient with NHYA class II HF. Due to increased risk of acute kidney injury and hyperkalemia, serum creatinine should be >2.5 mg in men at baseline with a potassium of >5.0 mEq/L for spironolactone use. Eplerenone is contraindicated in men with a serum creatinine >2 mg/dL and should be dose adjusted if potassium rises above 5.4 mEq/L. These laboratory results should be monitored continually throughout therapy with these agents. In patients with diabetes mellitus and a left ventricular EF (LVEF) of 40% or less who have recently experienced and acute MI and develop symptoms of HF, the use of an aldosterone antagonist has been shown to decrease morbidity and mortality. One limiting adverse event that can occur in men is gynecomastia. Occurrence of this adverse event can contribute to nonadherence of aldosterone antagonists. Target dosing guidelines for spironolactone and eplerenone are provided in TABLE 3.2
Hydralazine/isosorbide Dinitrate: In African American patients with NYHA class III or IV HF who remain symptomatic despite GDMT, hydralazine in combination with isosorbide dinitrate has been shown to decrease morbidity and mortality. Further data are required to determine the benefit of this combination in non–African Americans. The target total daily dose of hydralazine is 225 mg and that of isosorbide is 120 mg divided into three daily doses. This frequent daily dosing can negatively impact adherence, as do the adverse events. Headache, dizziness, and gastrointestinal issues are the most frequently reported adverse events with hydralazine/isosorbide dinitrate. Slower dosage titration can decrease the frequency of these issues.2
Ivabradine: Ivabradine has a very specific patient benefit population. Patients must have the following: 1) NYHA class II or III HF; 2) an LVEF £35%; 3) be on GDMT; 4) be in sinus rhythm; and 5) have a resting heart rate ³70 beats per minute. Patients meeting these criteria in the SHIFT trial showed a decrease in death from CV causes and HF hospitalization.9 It is important that BBs are increased to target doses with tolerable heart rates prior to the addition of ivabradine, as BBs have a higher body of evidence surrounding mortality reduction. Target dosing of ivabradine is 7.5 mg daily (TABLE 3). Heart rate is the most important monitoring parameter for patients taking ivabradine.7
Digoxin: Use of digoxin in patients with HFrEF has been shown to decrease HF hospitalizations and improve symptom control, exercise tolerance, and health-related quality of life. Patients with an uncorrected heart block should not be started on digoxin. Lower doses of digoxin (0.125 mg) should be utilized as a starting dose or when the patient is of advanced age or has poor kidney function. Doses higher than 0.25 mg are not seen in HF management. Digoxin levels (target 0.5-0.9 mg/mL) should be monitored, especially if digoxin toxicity is a concern. Adverse events associated with digoxin include arrhythmias, gastrointestinal symptoms, and neurologic complications. It is important to screen for drug interactions that can increase the risk of digoxin toxicity.2
Stage D HF is managed with advanced treatment strategies and procedures to manage fluid balance, transplantation, and end-of-life care.2
Drugs Beyond the Guidelines
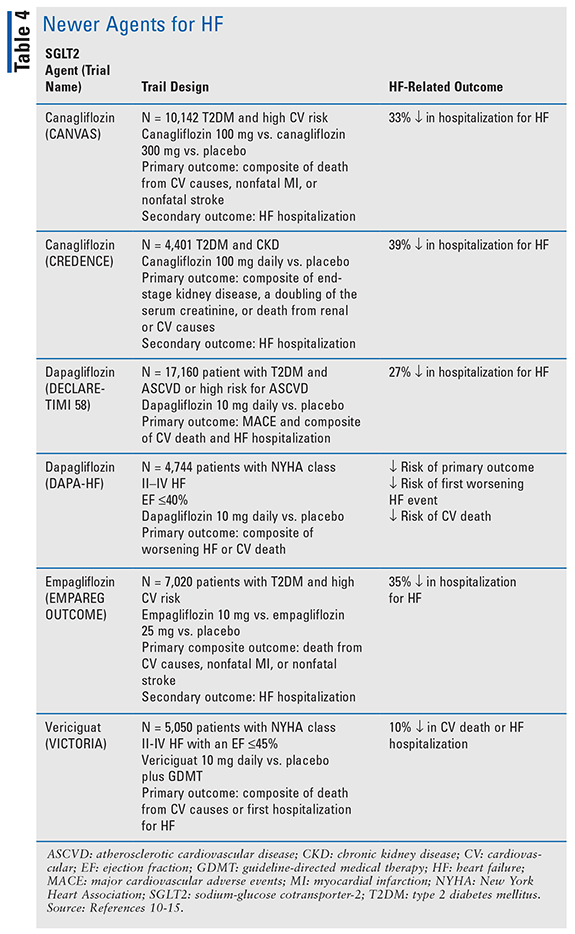
Since the update to the guideline in 2017, data have come out showing HF benefit for several other agents. Several of the sodium-glucose cotransporter- 2 inhibitors (SGLT-2is) have shown to decrease HF hospitalizations (TABLE 4).10-13 In the DAPA-HF trial, a decrease in the risk of CV death, first worsening HF event, and HF hospitalizations was seen.14 This was the first trial in SGLT-2is in which the primary outcome was related to HF instead of diabetes.14 This trial came about after several other clinical trials examining SGLT-2s showed a decrease in HF exacerbations when they are used for diabetes management (TABLE 4).10-13 The VICTORIA trial, which was published in 2020, examined the use of a new agent vericiguat (Verquvo) in HF. Vericiguat is an oral soluble guanylate cyclase stimulator, thought to enhance the effect of nitric oxide and increase cGMP activity. Vericiguat 10 mg daily showed a 10% decrease in the primary outcome compared with placebo, which was a composite of death from CV causes or first hospitalization for HF.15
Adherence Strategies
Each admission to the hospital for HF results in increases in cost, resource utilization, morbidity, and mortality. One study showed that 83% of patients with HF were hospitalized at least once, while 43% reported four admissions.1 Transitions of care positively impact hospital readmissions. As HF patients take multiple medications, poor adherence is associated with an increased risk of readmission.16 Poor adherence is also positively associated with lower health literacy. Pharmacists have been shown to improve medication adherence in HF patients through patient education and communication with other healthcare providers.17
With the recent increase in new therapies, poor adherence remains a significant obstacle to improving outcomes, including the increased risk for readmission and mortality.17 As former Surgeon General C. Everett Koop put it, “Drugs don’t work in patients who don’t take them.” Adherence issues in patients with HF do not only surround medications but are a concern in self-care recommendations as well. Self-care is important, and a lack thereof may also contribute to readmission risk.18 Pharmacists may continue to impact adherence and HF outcomes by emphasizing adherence through education, monitoring for early signs of nonadherence, and proactive communication with other healthcare team members.19
Role of the Health System Pharmacist
Study after study continues to show that having a pharmacist member of a team helps improve outcomes for HF patients.20-22 Pharmacists are uniquely positioned to participate in medication reconciliation to help prevent medication duplications or omissions, avoid the use of medications that could lead to HF exacerbations or drug interactions, and assess the use of complementary alternative medicine (CAM) that may not otherwise be addressed.23-25 As HF patients are on many medications, they are at risk for duplication of therapy, drug interactions, and adverse drug reactions that could lead to readmission or increased hospital stay. Pharmacist involvement in medication reconciliation and transitions of care in high-risk HF patients is becoming the standard.26 During an inpatient admission, visit to a clinic, or trip to the community pharmacy, opportunities are present for a pharmacist to provide patient education, assess for improper medication use such as nonsteroidal anti-inflammatory drugs or calcium channel blockers, which could exacerbate HF, ensure medications are being titrated to GDMT target dosing, assess for adverse drug reactions, and evaluate any OTC medications or CAM that the patient may be taking to ensure appropriateness. The goal of this is to avoid HF exacerbations.
Target doses of BBs, ACEIs, and ARBs are routinely not achieved. Often, patients are even started on additional GDMT without first attaining target BB or ACEI/ARB dosing, which has been shown to have a dose-related benefit to mortality in HFrEF.19 Pharmacists have demonstrated a positive impact on target HF therapy dose achievement.27,28 Along with patient monitoring and GDMT recommendations, pharmacists can bring awareness to new HF treatments, educate patients and providers, and contribute to transitions of care, including medication reconciliation and postdischarge follow-up.29
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
1. Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke Statistics—2021 update: a report from the American Heart Association. Circulation. 2021;143: e254-e743.
2. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-e239.
3. Steinberg BA, Zhao X, Heidenreich PA, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126:65-75.
4. Chang PP, Wruck LM, Shahar E, et al. Trends in hospitalizations and survival of acute decompensated heart failure in four US communities (2005–2014). Circulation. 2018;138:12-24.
5. Folsom AR, Shah AM, Lutsey PL, et al. American Heart Association’s Life’s Simple 7: avoiding heart failure and preserving cardiac structure and function. Am J Med. 2015;128(9):970-976.
6. American Heart Association. My Life Check–Life’s Simple 7. www.heart.org/en/healthy-living/healthy-lifestyle/my-life-check--lifes-simple-7. Accessed April 11, 2021.
7. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6):776-803.
8. McMurray JV, Packer M, Desai AS, et al. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993-1004.
9. Swedberg K, Komajda M, Bohm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376(9744):875-885.
10. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657.
11. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019; 380:2295-2306.
12. Wiviott SD, Raz I, Bonaca M, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
13. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
14. McMurry JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995-2008.
15. Armstrong PW, Pieske B, Anstrom KJ, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020;382:1883-1893.
16. Ruppar TM, Cooper PS, Mehr DR, et al. Medication adherence interventions improve heart failure mortality and readmission rates: systematic review and meta-analysis of controlled trials. J Am Heart Assoc. 2016;5(6):e002606.
17. Pallangyo P, Millinga J, Bhalia S, et al. Medication adherence and survival among hospitalized heart failure patients in a tertiary hospital in Tanzania: a prospective cohort study. BMC Res Notes. 2020;13:89.
18. Seid MA, Abdela OA, Zeleke EG. Adherence to self-care recommendations and associat ed factors among heart failure patients. From the patient’s point of view. PLoS One. 2019;14(2):e0211768.
19. Peri-Okonny P, Mi X, Khariton Y, et al. Target doses of heart failure medical therapy and blood pressure: insights from the CHAMP-HF Registry. JACC Heart Fail. 2019;7(4):350-358.
20. Martinez AS, Saef J, Paszczuk A, Bhatt-Chugani H. Implementation of a pharmacist-managed heart failure medication titration clinic. Am J Health Syst Pharm. 2013;70:1070-1076.
21. Murray MD, Young J, Hoke S, et al. Pharmacist intervention to improve medication adherence in heart failure: a randomized trial. Ann Intern Med. 2007;146:714-725.
22. Warden BA, Freels JP, Furuno JP, Mackay J. Pharmacy-managed program for providing education and discharge instructions for patients with heart failure. Am J Health Syst Pharm. 2014;71:134-139.
23. Christensen M, Lundh A. Medication review in hospitalised patients to reduce morbidity and mortality. Cochrane Database Syst Rev. 2013;2:CD008986
24. Ryan R, Santesso N, Hill S, et al. Consumer-oriented interventions for evidence-based prescribing and medicines use: an overview of systematic reviews. Cochrane Database Syst Rev. 2011;5:CD007768.
25. Eggink RN, Lenderink AW, Widdershoven JW, van den Bemt PM. The effect of a clinical pharmacist discharge service on medication discrepancies in patients with heart failure. Pharm World Sci. 2010;32(6):759-766.
26. Cheng JW. Current perspectives on the role of the pharmacist in heart failure management. Integ Pharm Res Pract. 2018;7:1-11.
27. Noschese LA, Bergman CL, Brar CK, Kansal MM. The pharmacist’s role in medication optimization for patients with chronic heart failure. Fed Pract. 2017;34(Suppl 10):S10-S15.
28. Casey T. Pharmacist intervention with heart failure patients. First Report Managed Care. 2012;9(1).
29. Anderson SL, Marrs JC. A review of the role of the pharmacist in heart failure transition of care. Adv Ther. 2018;35:311-323.
To comment on this article, contact rdavidson@uspharmacist.com.





